- Root nodule
-
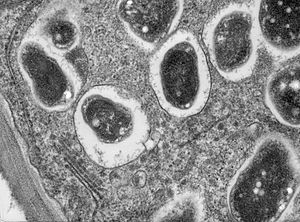 Cross section though a soybean (Glycine max 'Essex') root nodule. The bacterium, Bradyrhizobium japonicum, colonizes the roots and establishes a nitrogen fixing symbiosis. This high magnification image shows part of a cell with single bacteroids within their symbiosomes. In this image, endoplasmic reticulum, dictysome and cell wall can be seen.
Cross section though a soybean (Glycine max 'Essex') root nodule. The bacterium, Bradyrhizobium japonicum, colonizes the roots and establishes a nitrogen fixing symbiosis. This high magnification image shows part of a cell with single bacteroids within their symbiosomes. In this image, endoplasmic reticulum, dictysome and cell wall can be seen.
Root nodules occur on the roots of plants (primarily Fabaceae) that associate with symbiotic nitrogen-fixing bacteria. Under nitrogen-limiting conditions, capable plants form a symbiotic relationship with a host-specific strain of bacteria known as rhizobia. This process has evolved multiple times within the Fabaceae, as well as in other species found within the Rosid clade.[1]
Within legume nodules, nitrogen gas from the atmosphere is converted into ammonia, which is then assimilated into amino acids (the building blocks of proteins), nucleotides (the building blocks of DNA and RNA as well as the important energy molecule ATP), and other cellular constituents such as vitamins, flavones, and hormones. Their ability to fix gaseous nitrogen makes legumes an ideal agricultural organism as their requirement for nitrogen fertilizer is reduced. Indeed high nitrogen content blocks nodule development as there is no benefit for the plant of forming the symbiosis. The energy for splitting the nitrogen gas in the nodule comes from sugar that is translocated from the leaf (a product of photosynthesis). Malate as a breakdown product of sucrose is the direct carbon source for the bacteroid. Nitrogen fixation in the nodule is very oxygen sensitive. Legume nodules harbor an iron containing protein called leghaemoglobin, closely related to animal myoglobin, to facilitate the conversion of nitrogen gas to ammonia.
Contents
Classification
Two main types of nodule have been described: determinate and indeterminate.[2]
Determinate nodules are found on tropical (sub)legumes, such as those of the genera Glycine (soybean), Phaseolus (common bean), Lotus, and Vigna. Determinate nodules lose meristematic activity shortly after initiation, thus growth is due to cell expansion resulting in mature nodules which are spherical in shape.
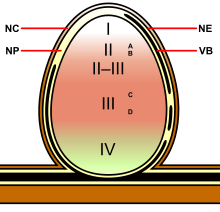
Indeterminate nodules are found on temperate legumes like Pisum (pea), Medicago (alfalfa), Trifolium (clover), and Vicia (vetch). They earned the moniker "indeterminate" because they maintain an active apical meristem that produces new cells for growth over the life of the nodule. This results in the nodule having a generally cylindrical shape. Because they are actively growing, indeterminate nodules manifest zones which demarcate different stages of development/symbiosis:[3][4][5]
- Zone I—the active meristem. This is where new nodule tissue is formed which will later differentiate into the other zones of the nodule.
- Zone II—the infection zone. This zone is permeated with infection threads full of bacteria. The plant cells are larger than in the previous zone and cell division is halted.
- Interzone II–III—Here the bacteria have entered the plant cells, which contain amyloplasts. They elongate and begin terminally differentiating into symbiotic, nitrogen-fixing bacteroids.
- Zone III—the nitrogen fixation zone. Each cell in this zone contains a large, central vacuole and the cytoplasm is filled with fully differentiated bacteroids which are actively fixing nitrogen. The plant provides these cells with leghemoglobin, resulting in a distinct pink color.
- Zone IV—the senescent zone. Here plant cells and their bacteroid contents are being degraded. The breakdown of the heme component of leghemoglobin results in a visible greening at the base of the nodule.
Nodulation
Legumes release compounds called flavonoids from their roots, which trigger the production of nod factors by the bacteria. When the nod factor is sensed by the root, a number of biochemical and morphological changes happen: cell division is triggered in the root to create the nodule, and the root hair growth is redirected to wind around the bacteria multiple times until it fully encapsulates 1 or more bacteria. The bacteria encapsulated divide multiple times, forming a microcolony. From this microcolony, the bacteria enter the developing nodule through a structure called an infection thread, which grows through the root hair into the basal part of the epidermis cell, and onwards into the root cortex; they are then surrounded by a plant-derived membrane and differentiate into bacteroids that fix nitrogen.
Nodulation is controlled by a variety of processes, both external (heat, acidic soils, drought, nitrate) and internal (autoregulation of nodulation, ethylene). Autoregulation of nodulation controls nodule numbers per plant through a systemic process involving the leaf. Leaf tissue senses the early nodulation events in the root through an unknown chemical signal, then restricts further nodule development in newly developing root tissue. The Leucine rich repeat (LRR) receptor kinases (NARK in soybean (Glycine max); HAR1 in Lotus japonicus, SUNN in Medicago truncatula) are essential for autoregulation of nodulation (AON). Mutation leading to loss of function in these AON receptor kinases leads to supernodulation or hypernodulation. Often root growth abnormalities accompany the loss of AON receptor kinase activity, suggesting that nodule growth and root development are functionally linked. I. Investigations into the mechanisms of nodule formation showed that the ENOD40 gene, coding for a 12–13 amino acid protein [41], is up-regulated during nodule formation [3].
Connection to root structure
Root nodules apparently have evolved three times within the Fabaceae but are rare outside that family. The propensity of these plants to develop root nodules seems to relate to their root structure. In particular, a tendency to develop lateral roots in response to abscisic acid may enable the later evolution of root nodules.[6]
In other species
Main article: Actinorhizal plantRoot nodules that occur on non-legume genera like Parasponia in association with Rhizobium bacteria, and those that arise from symbiotic interactions with Actinobacteria Frankia in some plant genera such as Alnus, vary significantly from those formed in the legume-rhizobia symbiosis. In these symbioses the bacteria are never released from the infection thread. Frankia nodulates approximately two hundred species in the following orders (families in parentheses): Cucurbitales (Coriariaceae and Datiscaceae), Fagales (Betulaceae, Casuarinaceae, and Myricaceae), Rosales (Rhamnaceae, Elaeagnaceae and Rosaceae).[7] Actinorhizal symbioses account for roughly the same amount of nitrogen fixation as rhizobial symbioses.[7]
Some fungi produce nodular structures known as tuberculate ectomycorrhizae on the roots of their plant hosts. Suillus tomentosus, for example, produces these structures with its plant host lodgepole pine (Pinus contorta var. latifolia). These structures have in turn been shown to host nitrogen fixing bacteria which contribute a significant amount of nitrogen and allow the pines to colonize nutrient-poor sites.[8]
References
- ^ Doyle, J. J., & Luckow, M. A. (2003). "The Rest of the Iceberg. Legume Diversity and Evolution in a Phylogenetic Context". Plant Physiology 131 (3): 900–910. doi:10.1104/pp.102.018150. PMC 1540290. PMID 12644643. http://www.plantphysiol.org/cgi/content/full/131/3/900.
- ^ Martin Crespi and Susana Gálvez (2000). "Molecular Mechanisms in Root Nodule Development". Journal of Plant Growth and Regulation 19 (2): 155–166. doi:10.1007/s003440000023. PMID 11038225. http://www.springerlink.com/content/2y6pbrdwqtegml7c/fulltext.pdf.
- ^ Fabrice Foucher and Eva Kondorosi (2000). "Cell cycle regulation in the course of nodule organogenesis in Medicago". Plant Molecular Biology 43 (5–6): 773–786. doi:10.1023/A:1006405029600. PMID 11089876. http://www.springerlink.com/content/v82q0x2475624q10/fulltext.pdf.
- ^ Hannah Monahan-Giovanelli, Catalina Arango Pinedo, and Daniel J. Gage (2006). "Architecture of Infection Thread Networks in Developing Root Nodules Induced by the Symbiotic Bacterium Sinorhizobium meliloti on Medicago truncatula". Plant Physiology 104 (2): 661–670. doi:10.1104/pp.105.072876. PMC 1361332. PMID 16384905. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1361332.
- ^ Willem Van de Velde , Juan Carlos Pérez Guerra , Annick De Keyser , Riet De Rycke , Stéphane Rombauts , Nicolas Maunoury , Peter Mergaert , Eva Kondorosi , Marcelle Holsters, and Sofie Goormachtig (2006). "Aging in Legume Symbiosis. A Molecular View on Nodule Senescence in Medicago truncatula". Plant Physiology Review 141 (2): 711–20. doi:10.1104/pp.106.078691. PMC 1475454. PMID 16648219. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1475454.
- ^ Yan Liang and Jeanne M. Harris (2005). "Response of root branching to abscisic acid is correlated with nodule formation both in legumes and nonlegumes". American Journal of Botany 92 (10): 1675–1683. doi:10.3732/ajb.92.10.1675. http://www.amjbot.org/cgi/content/full/92/10/1675.
- ^ a b Jeff J. Doyle (1998). "Phylogenetic perspectives on nodulation: evolving views of plants and symbiotic bacteria". Trends in Plant Science 3 (12): 473–778. doi:10.1016/S1360-1385(98)01340-5. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TD1-3VCVDPB-C&_user=456938&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId=997369551&_rerunOrigin=google&_acct=C000021830&_version=1&_urlVersion=0&_userid=456938&md5=340cae9b30de0b827c712df9daad138c.
- ^ Paul, L.R.; Chapman, B.K.; Chanway, C.P. (2007). "Nitrogen Fixation Associated with Suillus tomentosus Tuberculate Ectomycorrhizae on Pinus contorta var. latifolia". Annals of Botany 99: 1101–1109. doi:10.1093/aob/mcm061. http://aob.oxfordjournals.org/content/99/6/1101.full.
External links
Categories:- Plant roots
- Plant physiology
- Legumes
- Nitrogen metabolism
- Symbiosis
- Oligotrophs
Wikimedia Foundation. 2010.