- Bismuth subgallate
-
Bismuth subgallate 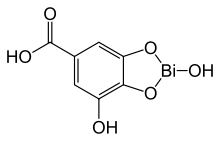
Systematic (IUPAC) name 2,7-dihydroxy-1,3,2-benzodioxabismole-5-carboxylic acid Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status OTC (US) Identifiers CAS number 99-26-3 
ATC code A07BB PubChem CID 16682999 ChemSpider 10607905 
UNII YIW503MI7V 
ChEBI CHEBI:31292 
Chemical data Formula C7H5BiO6 Mol. mass 394.091 g·mol−1 SMILES eMolecules & PubChem Physical data Density 1.1 g/cm³  (what is this?) subgallate (verify)
(what is this?) subgallate (verify)Bismuth subgallate, with a chemical formula C7H5BiO6, is the active ingredient in Devrom (internal deodorant), an over-the-counter FDA-approved medicine commonly used to treat malodor by deodorizing flatulence and stool. Also, it has been used to treat Helicobacter pylori infection and is used in wound therapy. As an internal deodorant, it is commonly used by individuals who have had ostomy surgery, bariatric surgery, fecal incontinence, and irritable bowel syndrome.[1]
It can cause darkening of the tongue and stools, which is temporary and harmless. A reversible encephalopathy was noted and examined in a few subjects taking bismuth subgallate.[2]
Contents
Safety
It is quite nontoxic, but it may cause minor irritation.
See also
External links
- American Cancer Society: Ileostomy Guide [1]
- Cleveland Clinic-Having an Ileostomy– A Primer for New Ostomates [2]
- United Ostomy Association of America-Ileostomy Guide [3]
- The Ostomy Files:The Issue of Oral Medications and a Fecal Ostomy [4]
- Devrom website [5]
References
- ^ Gorbach S. L. (1990). "Bismuth therapy in gastrointestinal diseases". Gastroenterology 99 (3): 863–75. PMID 2199292.
- ^ Burns R., Thomas D. W., Barron V. J. (1974). "Reversible encephalopathy possibly associated with bismuth subgallate ingestion". British Medical Journal 9 (1): 220–3. PMC 1633100. PMID 4818163. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1633100.
Categories:- Drugs acting on the gastrointestinal system and metabolism
- Bismuth heterocycles
Wikimedia Foundation. 2010.
