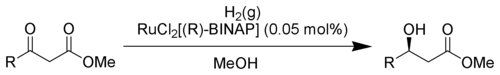
Noyori asymmetric hydrogenation
- Noyori asymmetric hydrogenation
-
The Noyori asymmetric hydrogenation is a chemical reaction described as an asymmetric reduction of β-keto-esters.[1][2]
Both enantiomers of BINAP are commercially available and widely used. Additionally, both BINAP enantiomers can be prepared from (±)-1,1'-bi-2-naphthol.[3]
Several reviews have been published.[4][5]
Ryōji Noyori discovered this catalytic reduction system in 1987 and shared half of the Nobel Prize in Chemistry in 2001 with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the Prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions (Sharpless epoxidation).
References
- ^ Noyori, R., Okhuma, T.; Kitamura, M.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akuragawa, S. J. Am. Chem. Soc. 1987, 109, 5856–5858. (doi:10.1021/ja00253a051)
- ^ Kitamura, M.; Tokunaga, M.; Ohkuma, T.; Noyori, R. Org. Syn., Coll. Vol. 9, p.589 (1998); Vol. 71, p.1 (1993). (Article)
- ^ Takaya, H.; Akutagawa, S.; Noyori, R. Org. Syn., Coll. Vol. 8, p.57 (1993); Vol. 67, p.20 (1989). (Article)
- ^ Noyori, Ryoji (1994). Asymmetric Catalysis In Organic Synthesis. Wiley-Interscience. ISBN 0-471-57267-5.
- ^ Ager, D. J.; Laneman, S. A. Tetrahedron: Asymmetry 1997, 8, 3327–3355. (Review)
Categories:
- Organic redox reactions
- Name reactions
Wikimedia Foundation.
2010.
Look at other dictionaries:
Asymmetric catalytic reduction — is the use of various chiral catalysts to reduce a prochiral organic compound to obtain a chiral product. This is one of the several techniques used in chiral synthesis.Typically, a transition metal is used with a bulky chiral ligand to such that … Wikipedia
Hydrogenation — |date=1996|location=Washington, D.C.|pages=429|id=ISBN 0 8412 3344 6] Because of the importance of hydrogen, many related reactions have been developed for its use. Most hydrogenations use gaseous hydrogen (H2), but some involve the alternative… … Wikipedia
Asymmetric synthesis — Asymmetric synthesis, also called chiral synthesis, enantioselective synthesis or stereoselective synthesis, is organic synthesis which introduces one or more new and desired elements of chirality. [GoldBookRef|title=asymmetric synthesis| file =… … Wikipedia
Hydrogénation asymétrique de Noyori — L hydrogénation asymétrique de Noyori est une réaction chimique décrite comme une reduction asymmetrique d un β céto ester[1],[2] … Wikipédia en Français
Ryōji Noyori — Born 3 September 1938 (1938 09 03) (age 73) Kobe, Japan … Wikipedia
Ryoji Noyori — Infobox Scientist name = Ryoji Noyori image size = 180px birth date = birth date and age|1938|09|03|df=yes birth place = Kobe, Japan nationality = Japan field = Chemistry work institutions = alma mater = doctoral advisor = doctoral students =… … Wikipedia
Transfer hydrogenation — is the addition of hydrogen (H2; dihydrogen in inorganic and organometallic chemistry) to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of… … Wikipedia
Ryōji Noyori — Ryoji Noyori Ryōji Noyori (jap. 野依 良治, Noyori Ryōji; * 3. September 1938 in Kobe, Japan) ist ein japanischer Chemiker. Inhaltsverzeichnis 1 Leben und Werk … Deutsch Wikipedia
Curtin–Hammett principle — The Curtin–Hammett principle is a principle in chemical kinetics proposed by David Yarrow Curtin and Louis Plack Hammett. It states that, for a reaction that has a pair of reactive intermediates or reactants that interconvert rapidly (as is… … Wikipedia
Kinetic resolution — In kinetic resolution, two enantiomers show different reaction rates in a chemical reaction, thereby creating an excess of the less reactive enantiomer.[1] This excess goes through a maximum and disappears on full completion of the reaction.… … Wikipedia