- Chlorotoxin
-
Chlorotoxin 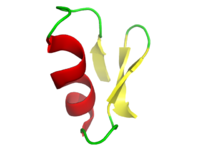 L-methionyl-L-cysteinyl-L-methionyl-L-prolyl-L-cysteinyl-L-phenylalanyl-L-threonyl-L-threonyl-L-.alpha.-aspartyl-L-histidyl-L-glutaminyl-L-methionyl-L-alanyl-L-arginyl-L-lysyl-L-cysteinyl-L-α-aspartyl-L-α-aspartyl-L-cysteinyl-L-cysteinylglycylglycyl-L-lysylglycyl-L-arginylglycyl-L-lysyl-L-cysteinyl-L-tyrosylglycyl-L-prolyl-L-glutaminyl-L-cysteinyl-L-leucyl-L-cysteinyl-L-argininamide, cyclic (219),(528),(1633),(2035)-tetrakis(disulfide)Other namesMCMPCFTTDHQMARKCDDCCGGKGRGKCYGPQCLCR
L-methionyl-L-cysteinyl-L-methionyl-L-prolyl-L-cysteinyl-L-phenylalanyl-L-threonyl-L-threonyl-L-.alpha.-aspartyl-L-histidyl-L-glutaminyl-L-methionyl-L-alanyl-L-arginyl-L-lysyl-L-cysteinyl-L-α-aspartyl-L-α-aspartyl-L-cysteinyl-L-cysteinylglycylglycyl-L-lysylglycyl-L-arginylglycyl-L-lysyl-L-cysteinyl-L-tyrosylglycyl-L-prolyl-L-glutaminyl-L-cysteinyl-L-leucyl-L-cysteinyl-L-argininamide, cyclic (219),(528),(1633),(2035)-tetrakis(disulfide)Other namesMCMPCFTTDHQMARKCDDCCGGKGRGKCYGPQCLCRIdentifiers CAS number 163515-35-3 
Properties Molecular formula C158H249N53O47S11 Molar mass 3995.71 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chlorotoxin is a 36-amino acid peptide found in the venom of the deathstalker scorpion (Leiurus quinquestriatus) which blocks small-conductance chloride channels.[1] The fact that chlorotoxin binds preferentially to glioma cells has allowed the development of new methods, that still are under investigation, for the treatment and diagnosis of several types of cancer.[2]
Contents
Source
Chlorotoxin can be purified from crude leiurus, which belongs to scorpion toxin super family.[3]
Chemistry
Chlorotoxin is a small toxin and at pH 7 is highly positive charged. It is a peptide consisting of 30–35 amino acids and 4 disulfide bonds; eight cysteine amino acids among its total number of amino acids present in the structure of this peptide are connected with four disulfide bonds.[4] Chlorotoxin has a considerable sequence homology to the class of small insectotoxins.[3][5]
Target
Chlorotoxin is the first reported high-affinity peptide ligand for Cl- channels and it blocks small conductance chloride channels. Each chloride channel can be closed by only one ligand molecule.[1][3]
Using a recombinant chlorotoxin it was demonstrated that chlorotoxin specifically and selectively interacts with MMP-2 isoforms which are specifically upregulated in gliomas and related cancers, but are not normally expressed in brain.[2]
Toxicity
Chlorotoxin immobilizes the envenomated prey. Duration of paralysis depends on the amount of chlorotoxin injected. In crayfish, chlorotoxin at 1.23-2.23 µg/g body wt produced a loss of motor control beginning ±20 seconds after injection which progressed to a rigid paralysis of the walking and pincer legs that was complete within an additional ±40 seconds. Within ±90 s of injection, the tail musculature was immobilized. No recovery was noted for 6 hours, at which time the crayfish were destroyed. At 0.5µg/g, chlorotoxin induced the same progressive paralysis with a slower onset. Recovery of crayfish was noted after 2 hours. The injection on insects produced similar results to those observed in crayfish.[3]
Therapeutic use
The fact that chlorotoxin binds preferentially to glioma cells compared with non-neoplastic cells or normal brain has allowed the development of new methods for the treatment and diagnosis of several types of cancer.[6]
CLTX has the ability to interact with chloride channels in membrane protein in glioma cells, so this prevents transmembrane chloride fluxes but this interaction does not happen for the neurons and normal glial cells. This suggests a potential treatment cancer.[7]
A report showed the anti-invasive effect of chlorotoxin on glioma cells mediated by its interaction with MMP-2, which allows the penetration of normal and tumor cells through tissue barriers. Chlorotoxin exerts a dual effect on MMP-2: it inhibits the enzymatic activity of MMP-2 and causes a reduction in the surface expression of MMP-2. This result implies the use of chlorotoxin as a highly effective drug of therapeutic potential for diseases that involve the activity of MMP-2.[2]
TM-601 which is the synthetic version of chlorotoxin is under phase II clinical trial. Iodine-131-TM-601 is used to treat malignant glioma. TM-601 is also a candidate for targeting gliomas because it crosses blood-brain and tissue barriers and binds to malignant brain tumor cells without affecting healthy tissue.[8]
CTX: Cy5.5 bioconjugate which is a combination of chlorotoxin and a fluorescent material named, Cy5.5 has been recently used by Researchers at Seattle Children’s Hospital Research Institute and Fred Hutchinson Cancer Research Center to demarcate cancer cells from the surrounding normal cells.[9] This gives surgeons a better chance of removing all of the cancerous cells without injuring the surrounding healthy tissue. CTX: Cy5.5 is a fluorescent molecule emitting photons in the near infrared spectrum and hence can be visualized in the operating room with the aid of infrared glasses. Studies in mouse models have shown that Cy5.5 can identify tumors with as few as 2000 cancer cells, making it 500 times more sensitive than MRI. Treated animals exhibited no neurologic or behavioral deficits, and postmortem studies revealed no evidence of neuropathy.[10]
References
- ^ a b DeBin JA, Strichartz GR (1991). "Chloride channel inhibition by the venom of the scorpion Leiurus quinquestriatus". Toxicon 29 (11): 1403–8. doi:10.1016/0041-0101(91)90128-E. PMID 1726031.
- ^ a b c Deshane J, Garner CC, Sontheimer H (February 2003). "Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2". J. Biol. Chem. 278 (6): 4135–44. doi:10.1074/jbc.M205662200. PMID 12454020.
- ^ a b c d DeBin JA, Maggio JE, Strichartz GR (February 1993). "Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion". Am. J. Physiol. 264 (2 Pt 1): C361–9. PMID 8383429. http://ajpcell.physiology.org/cgi/pmidlookup?view=reprint&pmid=8383429.
- ^ Lippens G, Najib J, Wodak SJ, Tartar A (January 1995). "NMR sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels". Biochemistry 34 (1): 13–21. doi:10.1021/bi00001a003. PMID 7819188.
- ^ Wudayagiri R, Inceoglu B, Herrmann R, Derbel M, Choudary PV, Hammock BD (2001). "Isolation and characterization of a novel lepidopteran-selective toxin from the venom of South Indian red scorpion, Mesobuthus tamulus". BMC Biochem. 2: 16. doi:10.1186/1471-2091-2-16. PMC 64496. PMID 11782289. http://www.biomedcentral.com/1471-2091/2/16.
- ^ Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H (November 1998). "Use of chlorotoxin for targeting of primary brain tumors". Cancer Res. 58 (21): 4871–9. PMID 9809993. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=9809993.
- ^ Lyons SA, O'Neal J, Sontheimer H (August 2002). "Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin". Glia 39 (2): 162–73. doi:10.1002/glia.10083. PMID 12112367.
- ^ Mamelak AN, Rosenfeld S, Bucholz R, et al (August 2006). "Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma". J. Clin. Oncol. 24 (22): 3644–50. doi:10.1200/JCO.2005.05.4569. PMID 16877732.
- ^ Veiseh M, Gabikian P, Bahrami SB, et al (July 2007). "Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci". Cancer Res. 67 (14): 6882–8. doi:10.1158/0008-5472.CAN-06-3948. PMID 17638899.
- ^ "Tumor Painting Revolutionizes Fight Against Cancer". http://www.fhcrc.org/about/ne/news/2007/07/16/tumor_painting.html.
Categories:- Proteins
- Experimental cancer drugs
- Ion channel toxins
Wikimedia Foundation. 2010.
