- Wien's displacement law
-
"Wien's Law" redirects here. For the historical distribution law, see Wien's Distribution Law.
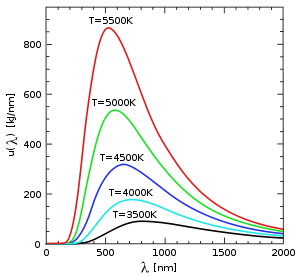 Black body thermal emission intensity as a function of wavelength for various absolute temperatures. Wien's law is not obvious in the picture, because the total emission includes a geometrical factor of 1/λ2 which counts the number of fourier modes of wavelength λ, and a second factor of 1/λ2 to convert intensities per-unit-frequency to intensities per-unit-wavelength
Black body thermal emission intensity as a function of wavelength for various absolute temperatures. Wien's law is not obvious in the picture, because the total emission includes a geometrical factor of 1/λ2 which counts the number of fourier modes of wavelength λ, and a second factor of 1/λ2 to convert intensities per-unit-frequency to intensities per-unit-wavelength
Wien's displacement law states that the wavelength distribution of thermal radiation from a black body at any temperature has essentially the same shape as the distribution at any other temperature, except that each wavelength is displaced on the graph. Apart from an overall T3 multiplicative factor, the average thermal energy in each mode with frequency ν only depends on the ratio ν / T. Restated in terms of the wavelength λ = c / ν, the distributions at corresponding wavelengths are related, where corresponding wavelengths are at locations proportional to 1 / T. Blackbody radiation approximates to Wien's law at high frequencies.
From this general law, it follows that there is an inverse relationship between the wavelength of the peak of the emission of a black body and its temperature when expressed as a function of wavelength, and this less powerful consequence is often also called Wien's displacement law in many textbooks.
where λmax is the peak wavelength, T is the absolute temperature of the black body, and b is a constant of proportionality called Wien's displacement constant, equal to 2.8977685(51)×10−3 m·K (2002 CODATA recommended value).
For wavelengths near the visible spectrum, it is often more convenient to use the nanometer in place of the meter as the unit of measure. In this case, b = 2,897,768.5(51) nm·K.
In the field of plasma physics temperatures are often measured in units of electron volts and the proportionality constant becomes b = 249.71066 nm·eV.
Contents
Explanation and familiar approximate applications
The law is named for Wilhelm Wien, who derived it in 1893 based on a thermodynamic argument. Wien considered adiabatic, or slow, expansion of a cavity containing waves of light in thermal equilibrium. He showed that under slow expansion or contraction, the energy of light reflecting off the walls changes in exactly the same way as the frequency. A general principle of thermodynamics is that a thermal equilibrium state, when expanded very slowly stays in thermal equilibrium. The adiabatic principle allowed Wien to conclude that for each mode, the adiabatic invariant energy/frequency is only a function of the other adiabatic invariant, the frequency/temperature.
Max Planck reinterpreted a constant closely related to Wien's constant b as a new constant of nature, now called Planck's constant, which relates the frequency of light to the energy of a light quantum.
Wien's displacement law implies that the hotter an object is, the shorter the wavelength at which it will emit most of its radiation, and also that the wavelength for maximal or peak radiation power is found by dividing Wien's constant by the temperature in kelvins.
Examples
- Light from the Sun and Moon: The effective temperature of the Sun is 5778 K. Using Wien's law, this temperature corresponds to a peak emission at a wavelength of 2.89777 million nm K/ 5778 K = 502 nm or about 5000 Å. This wavelength is fairly in the middle of the most sensitive part of land animal visual spectrum acuity. Even nocturnal animals must sense light from the moon, which is reflected sunlight with this same wavelength distribution. Also, the average wavelength of starlight maximal power is in this region, due to the sun being in the middle of a common temperature range of stars.
Wien's constant may be used in different units, and many examples to calculate familiar situation types of radiation required use of only one or two significant figures:
- Light from incandescent bulbs and fires: A lightbulb has a glowing wire with a somewhat lower temperature, resulting in yellow light, and something that is "red hot" is again a little less hot. It is easy to calculate that a wood fire at 1500 K puts out peak radiation at 3 million nm K /1500 K = 2000 nm = 20,000 Å. This is far more energy in the infrared than in the visible band, which ends about 7500 Å.
- Radiation from mammals and the living human body: Mammals at roughly 300 K emit peak radiation at 3 thousand μm K / 300 K = 10 μm, in the far infrared. This is therefore the range of infrared wavelengths that pit viper snakes and passive IR cameras must sense.
- The wavelength of radiation from the Big Bang: The blackbody radiation resulting from the Big Bang is also a typical application of Wien's law. Remembering that Wien's displacement constant is about 3 mm K, and the temperature of the Big Bang background radiation is about 3 K (actually 2.7 K), it is apparent that the microwave background of the sky peaks in power at 2.9 mm K / 2.7 K = just over 1 mm wavelength in the microwave spectrum. This provides a convenient rule of thumb for why microwave equipment must be sensitive on both sides of this frequency band, in order to do effective research on the cosmic microwave background.
Frequency-dependent formulation
In terms of frequency ν (in hertz), Wien's displacement law becomes
where α ≈ 2.821439... is a constant resulting from the numerical solution of the maximization equation, k is the Boltzmann constant, h is the Planck constant, and T is the temperature (in kelvins).
Because the spectrum from Planck's law of black body radiation takes a different shape in the frequency domain from that of the wavelength domain, the frequency location of the peak emission does not correspond to the peak wavelength using the simple relationship between frequency, wavelength, and the speed of light.
Derivation
Wilhelm Wien first derived this law in 1893 by applying the laws of thermodynamics to electromagnetic radiation.[1] A modern variant of Wien's derivation can be found in the textbook by Wannier.[2]
Wien noted that under adiabatic expansion, the energy of a mode of light, the frequency of the mode, and the total temperature of the light change together in the same way, so that their ratios are constant. This implies that in each mode at thermal equilibrium, the adiabatic invariant energy/frequency should only be a function of the adiabatic invariant frequency/temperature:
The form of F is now known from Planck's law:
Wien guessed the approximate pure exponential form, which is Wien's distribution law, a valid high frequency approximation to Planck's law. However, no matter what the function F is, the location of the peak of the distribution as a function of frequency is strictly proportional to T.
To get the usual expression for the blackbody curve, the energy per mode needs to be multiplied by the number density of modes with a given frequency ν:
so that this number density is proportional to the frequency squared. The total energy per unit frequency adds the ν2 modes together to give the total energy at frequency ν:
This per-unit-frequency expression for the density can be transformed to a per-unit-wavelength density by changing variables:
and since ν = c / λ, this adds a factor of
 :
:These different variables only introduce a power-law in front of the function F. For any function U of the form:
the location of the maximum or minimum of U is where the derivative is zero:
which yields the trivial solution xa − 1 = 0 and the equation:
which is an equation for x / T, so that the minima or maxima of U are at some definite value of x / T, at an x always strictly proportional to T. This is the peak displacement law: the peak location is proportional to the temperature whether the density is expressed in terms of wavenumber, in terms of frequency, in terms of (1/wavelength), or in terms of any other variable where the intensity only gets multiplied by a power of this variable.
The exact numerical location of the peak of the distribution depends on whether the distribution is considered per-mode-number, per-unit-frequency, or per-unit-wavelength, since the power law in front of F is different for the different forms.
Planck's law for the spectrum of black body radiation may be used to find the actual constant in the peak displacement law:
Differentiating u(λ,T) with respect to λ and setting the derivative equal to zero gives
which can be simplified to give
When the dimensionless quantity x is defined to be
then the equation above becomes
The numerical solution to this equation is[3]:
Solving for the wavelength λ in units of nanometers, and using kelvins for the temperature yields:
The frequency form of Wien's displacement law is derived using similar methods, but starting with Planck's law in terms of frequency instead of wavelength. The effective result is to substitute 3 for 5 in the equation for the peak wavelength. This is solved with x = 2.82143937212...
Using the value 4 in this equation (midway between 3 and 5) yields a "compromise" wavelength-frequency-neutral peak, which is given for x = 3.92069039487....
See also
- Sakuma–Hattori equation
- Stefan–Boltzmann law
References
- ^ Mehra, J.; Rechenberg, H. (1982). The Historical Development of Quantum Theory. New York: Springer-Verlag. Chapter 1. ISBN 9780387906423.
- ^ Wannier, G. H. (1987) [1966]. Statistical Physics. Dover Publications. Chapter 10.2. ISBN 9780486654010. OCLC 15520414.
- ^ The equation
 cannot be solved in terms of elementary functions. It can be solved in terms of Lambert's product log function but an exact solution is not important in this derivation.
cannot be solved in terms of elementary functions. It can be solved in terms of Lambert's product log function but an exact solution is not important in this derivation.
Further reading
- Soffer, B. H.; Lynch, D. K. (1999). "Some paradoxes, errors, and resolutions concerning the spectral optimization of human vision". American Journal of Physics 67 (11): 946–953. Bibcode 1999AmJPh..67..946S. doi:10.1119/1.19170.
- Heald, M. A. (2003). "Where is the 'Wien peak'?". American Journal of Physics 71 (12): 1322–1323. Bibcode 2003AmJPh..71.1322H. doi:10.1119/1.1604387.
External links
Categories:- Statistical mechanics
- Foundational quantum physics
- Optics
Wikimedia Foundation. 2010.


















