- Delépine reaction
-
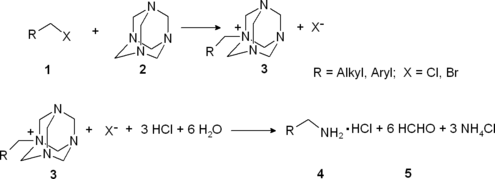
The Delépine reaction is the organic synthesis of primary amines (4) by reaction of a benzyl or alkyl halides (1) with hexamethylenetetramine (2) followed by acid hydrolysis of the quaternary ammonium salt (3).[1][2] It is named after the French chemist Stéphane Marcel Delépine (1871–1965).
Advantages of this reaction are selective access to the primary amine without side reactions from easily accessible reactants with short reaction times and relatively mild reaction conditions.
An example is the synthesis of 2-bromoallylamine from 2,3-dibromopropene.[3]
Reaction mechanism
The benzyl halide or alkyl halide 1 reacts with hexamethylenetetramine to a quaternary ammonium salt 3, each time just alkylating one nitrogen atom. By refluxing in concentrated ethanolic hydrochloric acid solution this salt is converted to the primary amine together with formaldehyde (as the acetal with ethanol) and ammonium chloride.
References
- ^ M. Delépine: Bull.Soc.Chim.Fr.. 1895, 13, S. 352 - 361
- ^ Alexander R. Surrey: Name Reactions in Organic Chemistry. 2nd Edition, Academic Press, 1961
- ^ Albert T. Bottini, Vasu Dev, and Jane Klinck (1973), "2-Bromoallylamine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0121; Coll. Vol. 5: 121
Categories:- Substitution reactions
- Name reactions
Wikimedia Foundation. 2010.