- BEZ235
-
BEZ235 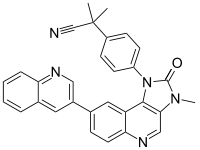 2-Methyl-2-{4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl]phenyl}propanenitrileOther namesNVP-BEZ235; BEZ-235
2-Methyl-2-{4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl]phenyl}propanenitrileOther namesNVP-BEZ235; BEZ-235Identifiers CAS number 915019-65-7 
PubChem 11977753 ChemSpider 10151099 
Jmol-3D images Image 1 - N#CC(c6ccc(N5c4c(cnc3ccc(c1cc2ccccc2nc1)cc34)N(C5=O)C)cc6)(C)C
- InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3

Key: JOGKUKXHTYWRGZ-UHFFFAOYSA-N
InChI=InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3
Properties Molecular formula C30H23N5O Molar mass 469.54 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references BEZ235 or NVP-BEZ235 is an imidazoquinoline derivative and PI3K inhibitor[1] being investigated as a possible cancer treatment.[2]
It has been shown to be toxic to Waldenström's macroglobulinemia cells.[3]
It was the first PI3K inhibitor to enter clinical trials, in 2006 (primary outcome results due in 2010).[4]
References
- ^ "NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas". http://mct.aacrjournals.org/content/8/8/2204.abstract.
- ^ Maira et al. (2009). "PI3K inhibitors for cancer treatment: where do we stand?". doi:10.1042/BST0370265. http://www.biochemsoctrans.org/bst/037/0265/0370265.pdf.
- ^ Sacco, A; Roccaro, A; Ghobrial, IM (2010). "Role of dual PI3/Akt and mTOR inhibition in Waldenstrom's Macroglobulinemia". Oncotarget 1 (7): 578–82. PMID 21317453.
- ^ "A Phase I/II Study of BEZ235 in Patients With Advanced Solid Malignancies Enriched by Patients With Advanced Breast Cancer". http://clinicaltrials.gov/ct2/show/NCT00620594.

This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it.
