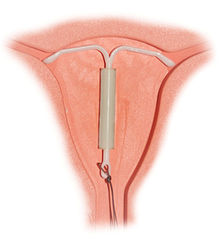
- IUD with progestogen
-
IntraUterine System (Mirena) Background Birth control type Intrauterine First use 1990 (Mirena - currently available)
1976 (Progestasert - discontinued in 2001)Failure rates (first year, Mirena) Perfect use 0.2% Typical use 0.2% Usage Duration effect 5 years Reversibility 2-6 months User reminders Check thread position monthly Clinic review Annually Advantages and disadvantages STD protection No Periods Causes menstrual irregularity, periods are usually lighter or none at all (amenorrhea) Weight No proven effect Benefits No need to remember to take any daily action Risks Ovarian cysts (usually benign)
Small risk PID, uterine perforationMedical notes (As for IUDs) when control heavy periods also required. The IUD with progestogen, intrauterine system (IUS), or IntraUterine Contraceptive (IUC) is a long-acting reversible hormonal contraceptive device that is placed in the uterus. An IUS has a hormone cylinder that releases a progestin (a synthetic progestogen) called levonorgestrel. The only brand currently available is the T-frame LNG-20 IUS, marketed as Mirena by Bayer.
The term IUS is used in the United Kingdom to distinguish the hormonal intrauterine contraceptive from copper-based intrauterine devices (IUDs). In the United States, all intrauterine contraceptives are referred to with the term IUD.[1]
Contents
Clinical uses
- Contraception
- menorrhagia (heavy periods), endometriosis, chronic pelvic pain, dysmenorrhea, and anemia. In some cases, use of an IUS may prevent a need for a hysterectomy.[2][3][4]
Fitting
The IUS can only be fitted by a qualified medical practitioner. The device should be inserted according to the manufacturer's instructions using aseptic technique to avoid introduction of bacteria into the uterus. Antibiotics should be given before insertion to women at high risk for endocarditis (inflammation of the inner layer of the heart), but should not be used routinely.[5]
During the placement appointment, the cervix is dilated in order to sound (measure) the uterus and insert the IUS. Cervix dilation is uncomfortable and, for some women, painful. Doctors often advise women to take painkillers before the procedure to reduce pain and discomfort, and some may even use a local anaesthetic. Insertion may be more comfortable if done midcycle, when the cervix is naturally dilated.[6]
Once in place, the IUS is approved for birth control for up to 5 years. The cumulative 5-year pregnancy rate is estimated to be 0.7%.[7]
Mechanisms of birth control
The Mirena is intended to initially release a daily dose of 20 micrograms levonorgestrel (a progestin). No single mechanism accounts for the effectiveness of the IUS in preventing pregnancy; it has several effects on the reproductive system:
- Frequency of ovulation is reduced.[8]
- Cervical mucus is changed to obstruct passage of sperm through the cervix.[9]
- The presence of a foreign body in the uterus prompts the release of leukocytes and prostaglandins by the endometrium, substances that are hostile to both sperm and eggs.[10] Some physicians believe these substances are also hostile to very early embryos.[11]
Removal
In general, IUS removal is easiest if undertaken toward the end of a woman's period and involves a doctor or trained nurse's use of a pair of forceps to take hold of the IUS's thread and gently retracting it.
A "lost coil" occurs when the thread cannot be felt by a woman on routine checking and is not seen on speculum examination.[12] Various thread collector devices or simple forceps may then be used to try and grasp the device through the cervix.[13] In the rare cases when this is unsuccessful, an ultrasound scan may be arranged to check the position of the coil and exclude its perforation through into the abdominal cavity or its unrecognised previous expulsion. Hysteroscopy is very rarely needed.
After removal of the IUS, normal fertility is regained after a few months, with a near-normal 80% of women able to conceive within 12 months.
Contraindications
The WHO Medical Eligibility Criteria for Contraceptive Use and RCOG Faculty of Family Planning & Reproductive Health Care (FFPRHC) UK Medical Eligibility Criteria for Contraceptive Use list the following as conditions where insertion of a levonorgestrel IUS is not usually recommended or should not be used because of an unacceptable health risk.:[14][15] According to Dr. Ayman A. A. Ewies in an abstract published in Gynecological Endocrinology, 31 July 2009 [16] the hormonal releasal of the Mirena is publicized to have a local effect but he shows that elevated levels of levonorgestrel have been found globally in some women because of various factors including low metabolic rates. He also states that weight gain is one of the major side effects.
Conditions that represent a health risk if a levonorgestrel IUS is inserted:
- weight gain
- Pregnancy
- Postpartum puerperal sepsis
- Immediately after a septic abortion
- Before evaluation of unexplained vaginal bleeding suspected of being a serious condition
- Malignant gestational trophoblastic disease
- Cervical cancer (awaiting treatment)
- Active liver disease: (acute viral hepatitis, severe decompensated cirrhosis, benign or malignant liver tumours)
- Current or recent breast cancer[17]
- Endometrial cancer
- Current PID
- Current purulent cervicitis, chlamydial infection, or gonorrheal STIs
- Known pelvic tuberculosis
Conditions where the theoretical or proven risks usually outweigh the advantages of inserting a levonorgestrel IUS:
- Postpartum between 48 hours and 4 weeks (increased IUD expulsion rate with delayed postpartum insertion)
- Current deep vein thrombosis (DVT) or pulmonary embolus (PE)
- Benign gestational trophoblastic disease
- Ovarian cancer
- Very high individual likelihood of exposure to gonorrhea or chlamydial STIs
- Active liver disease: (acute viral hepatitis, severe decompensated cirrhosis, benign, or malignant liver tumours)
Side effects and complications
Location of device
Following insertion, the IUS may be expelled through the cervix. An expulsion rate of 4% was observed in the manufacturer's clinical trials, with most (3%) occurring in the first year of use. Expulsion is more common in younger women, women who have not had children, and when an IUS is inserted immediately after childbirth or abortion.[18][19][20]
A rare but potentially serious complication is that of uterine perforation. This may occur either during the device's insertion or from its later embedment into the myometrium (uterine wall) and subsequent migration through to the intra-abdominal cavity. Perforation can cause internal scarring, infection, or damage to other organs, and may require surgery. Uterine perforation has been reported at rates ranging from 1 to 2.6 per 1000 insertions. It is believed that perforations are significantly underreported, however, and actual perforation rates are likely higher.[21]
Both expulsion and perforation result in loss of contraceptive cover, and the position of the thread of the IUS should be self-checked at least once per month to verify that it is still in place.
The string(s) may be felt by some men during intercourse. If this is problematic, the provider may tuck the strings behind the cervix, cut the strings shorter, or in more extreme cases cut the strings to level with the cervix. Cutting the strings even with the cervix prevents the woman from checking the device's correct placement, and may complicate removal.
Pelvic inflammatory disease and sexually transmitted diseases
Pelvic inflammatory disease (PID) is caused by certain sexually transmitted diseases (STDs). PID is a serious condition that may result in infertility. IUDs can increase the risk of PID above baseline for the first 21 days after insertion and is related to the presence of an infection at the time of the insertion. STD testing should be offered at the time of an insertion. Current infection is not a reason to delay IUD insertion but if an infection is found after the insertion, the patient needs to be treated. The IUD does not need to be removed. 21 days after insertion the risk of PID returns to baseline for all women. [22]
An animal study suggested that progestin-only hormonal contraceptives such as Mirena might increase the risk of HIV transmission, because of the thinning of the vaginal walls caused by these methods.[23] However, a number of studies of human populations showed that progestin contraceptive use does not increase the risk of acquiring HIV.[24]
However, like the oral contraceptive pill and other non-barrier forms of contraception, the IUS offers no protection against sexually transmitted disease.
Postpartum and post-abortion insertion
An IUS may be inserted immediately postpartum (within 48 hours). With insertions after 48 hours, perforation of the uterus is more likely to occur when uterine involution is incomplete; involution usually completes by 4–6 weeks postpartum.[19] Special considerations apply to women who plan to breastfeed.
Also, to allow for uterine involution, insertion of an IUS is not recommended for women having had a D&E abortion (second-trimester abortion) within the past four weeks.[6] Post-abortion IUD is safe and effective and has only a slightly increased risk of expulsion compared to interval insertion.
To reduce the risk of infection, insertion of an IUS is not recommended for women that have had a medical abortion but have not yet had an ultrasound to confirm that the abortion was complete, or that have not yet had their first menstruation following the medical abortion.[6]
Expulsion is more common when an IUS is inserted immediately after childbirth or abortion.[19][20]
Hormonal side-effects
Localized
Menstrual periods become lighter or, in about 20% of women, stop completely within one year of insertion.[7] Irregular bleeding is common in the first few months after insertion, with the average user reporting 16 days of bleeding or spotting in the first month of use, but this diminishes to about four days at 12 months.[25][26]
Systemic
The progestin in an IUS is intended to be released at a lower dose than that used in other progestogen-only contraceptives such as the mini-pill or Norplant (blood levels of levonorgestrel in Mirena users are half those found in Norplant users and one-tenth those found in users of levonorgestrel-only pills).[27]
Enlarged follicles (ovarian cysts) have been diagnosed in about 12% of the subjects using a levonorgestrel IUS. Most of these follicles are asymptomatic, although some may be accompanied by pelvic pain or dyspareunia. In most cases the enlarged follicles disappear spontaneously during two to three months observation. Surgical intervention is not usually required.[7]
Nursing mothers
Progestogen-only contraceptives such as an IUS are not believed to affect milk supply or infant growth.[28] However, a study in the Mirena application for FDA approval found a lower continuation of breastfeeding at 75 days in IUS users (44%) versus copper IUD users (79%).[29]
When using Mirena®, about 0.1% of the maternal dose of levonorgestrel can be transferred via milk to the nursed infant.[30] A six-year study of breastfed infants whose mothers used a levonorgestrel-only method of birth control found the infants had increased risk of respiratory infections and eye infections, though a lower risk of neurological conditions, compared to infants whose mothers used a copper IUD.[31] No longer-term studies have been performed to assess the long-term effects on infants of levornogestrel in breast milk.
There are conflicting recommendations about use of Mirena while breastfeeding. The U.S. FDA does not recommend any hormonal method, including Mirena, as a first choice of contraceptive for nursing mothers.[citation needed] The World Health Organization recommends against immediate postpartum insertion, citing increased expulsion rates. It also reports concerns about potential effects on the infant's liver and brain development in the first six weeks postpartum. However, it recommends offering Mirena as a contraceptive option beginning at six weeks postpartum even to nursing women.[32] Planned Parenthood offers Mirena as a contraceptive option for breastfeeding women beginning at four weeks postpartum.[6]
Effect on cancer rates
The U.S. Food and Drug Administration has concluded that the carcinogenic potential of Mirena is low.[33] According to a 1999 evaluation of the studies performed on progestin-only birth control by the International Agency for Research on Cancer, there is some evidence that progestin-only birth control reduces the risk of endometrial cancer. The IARC concluded that there is no evidence progestin-only birth control increases the risk of any cancer, though the available studies were too small to be definitively conclusive.[34]
Levongestrel IUS is not recommended for women who have, have had, or suspect they have breast cancer.[7][17]
Pregnancy
Although it is one of the most effective methods of contraception, pregnancy may still occur. The risk of miscarriage or premature birth is increased for such pregnancies, the former particularly during the second trimester; these increased risks end if the IUD is removed after pregnancy is discovered.[35] No pattern of birth defects was found in the 35 babies for whom birth outcomes were available at the time of FDA approval.[36]
As many as half the pregnancies that occur in Mirena users may be ectopic. The incidence rate of ectopic pregnancies is approximately 1 per 1000 users per year.[37]
Bone Density
No evidence has been identified to suggest Mirena affects bone mineral density (BMD).[38] The only published study on the effect of Mirena on BMD showed that long-term users, at 7 years of use, had similar BMD at the midshaft of the ulna and at the distal radius as nonusers matched by age and BMI. In addition, BMD measurements were similar to the expected values for women in the same age group as the participants. The authors of the study said their results were predictable, since it is well established that the main factor responsible for bone loss in women is hypoestrogenism, and, in agreement with previous reports, they found estradiol levels in Mirena users to be normal.[39]
Types of IntraUterine Systems
Progestasert: 1976–2001
Progestasert was the first hormonal uterine device, developed in 1976[40] and manufactured until 2001. It contained progesterone that was released at a rate of 65 micrograms per day.[40] In most countries it was replaced annually, though it was approved for 18 months of use in France. It had a failure rate of 2% per year.[41]
Mirena: 1990–present
Development and studies of the Mirena Coil began in the 1970s.[42] Schering Health distributes Mirena outside the United States, while Berlex distributes it inside the United States. Both companies have worked with the Population Council, a non-profit organization that has worked with other contraceptive manufacturers (including Wyeth, maker of Norplant).
Mirena was first marketed commercially in Finland in 1990, but not approved by the U.S. Food and Drug Administration until 2000. It is intended to initially release 20 micrograms of levonorgestrel per day and may be used for five years.
Products in development
Femilis
Contrel, the Belgian company that developed the frameless GyneFix IUD, is developing a lower-dose (14 micrograms levonorgestrel per day) T-frame IUS named Femilis. Femilis would come in a smaller size (Femilis Slim) for nulliparous women. It would be inserted without a plunger, and it is hoped its performance would be less dependent on the experience of the health care professional.
FibroPlant-LNG
Several trials with positive results have been done on a frameless IUS called FibroPlant-LNG (also from Contrel). FibroPlant is anchored to the fundus of the uterus rather than being held in by a frame. It initially releases 14 micrograms of levonorgestrel per day, and may be used for at least three years. As of 2010, Fibroplant is available in Belgium from Dr Dirk Wildemeersch, the original researcher at Contrel, who carries out fittings at his office in Ghent.[43]
See also
References
- ^ Planned Parenthood - Intrauterine Devices: national health care provider describes Paragard and Mirena as IUDs.
- ^ Petta C, Ferriani R, Abrao M, Hassan D, Rosa E Silva J, Podgaec S, Bahamondes L (2005). "Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis.". Hum Reprod 20 (7): 1993–8. doi:10.1093/humrep/deh869. PMID 15790607.
- ^ Marjoribanks J, Lethaby A, Farquhar C (2006). Marjoribanks, Jane. ed. "Surgery versus medical therapy for heavy menstrual bleeding.". Cochrane Database Syst Rev (2): CD003855. doi:10.1002/14651858.CD003855.pub2. PMID 16625593.
- ^ Faundes A, Alvarez F, Brache V, Tejada A (1988). "The role of the levonorgestrel intrauterine device in the prevention and treatment of iron deficiency anemia during fertility regulation". Int J Gynaecol Obstet 26 (3): 429–33. doi:10.1016/0020-7292(88)90341-4. PMID 2900174.
- ^ IUDs—An Update. "Procedures for Providing IUDs". http://www.infoforhealth.org/pr/b6/B6boxes.shtml#procedures.
- ^ a b c d "Understanding IUDs". Planned Parenthood. July 2005. Archived from the original on 2006-10-12. http://web.archive.org/web/20061012053613/http://www.plannedparenthood.org/birth-control-pregnancy/birth-control/intrauterine-devices.htm. Retrieved 2006-10-08.
- ^ a b c d Bayer (2007). "Mirena U.S. Product Information". http://berlex.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf. Retrieved 2007-05-04.
- ^ FDA Medical Review p.36
- ^ FDA Medical Review p.12
- ^ Ortiz M, Croxatto H (1987). "The mode of action of IUDs". Contraception 36 (1): 37–53. doi:10.1016/0010-7824(87)90060-6. PMID 3311625.
- ^ Stanford J, Mikolajczyk R (2002). "Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects". Am J Obstet Gynecol 187 (6): 1699–708. doi:10.1067/mob.2002.128091. PMID 12501086., which cites:
- Smart Y, Fraser I, Clancy R, Roberts T, Cripps A (1982). "Early pregnancy factor as a monitor for fertilization in women wearing intrauterine devices". Fertil Steril 37 (2): 201–4. PMID 6174375.
- ^ Nijhuis J, Schijf C, Eskes T (1985). "The lost IUD: don't look too far for it". Ned Tijdschr Geneeskd 129 (30): 1409–10. PMID 3900746.
- ^ Kaplan N (1976). "Letter: Lost IUD". Obstet Gynecol 47 (4): 508–9. PMID 1256735.
- ^ WHO (2004). "Intrauterine devices (IUDs)". Medical Eligibility Criteria for Contraceptive Use (3rd ed.). Geneva: Reproductive Health and Research, WHO. ISBN 92-4-156266-8. http://www.who.int/reproductive-health/publications/mec/iuds.html.
- ^ FFPRHC (2006). "The UK Medical Eligibility Criteria for Contraceptive Use (2005/2006)" (PDF). Archived from the original on 2007-06-19. http://web.archive.org/web/20070619230102/http://www.ffprhc.org.uk/admin/uploads/UKMEC200506.pdf. Retrieved 2007-05-04.
- ^ Ayman A.A. Ewiess. "Mirena® - the Discontinuing Story". http://www.cbgnetwork.org/3538.html.
- ^ a b Trinh, X.; Tjalma, W.; Makar, A.; Buytaert, G.; Weyler, J.; Van Dam, P. (2008). "Use of the levonorgestrel-releasing intrauterine system in breast cancer patients". Fertility and sterility 90 (1): 17–22. doi:10.1016/j.fertnstert.2007.05.033. PMID 17706209.
- ^ IUDs—An Update. "Chapter 2.7:Expulsion". http://www.infoforhealth.org/pr/b6/b6chap2_7.shtml#top.
- ^ a b c IUDs—An Update. "Chapter 3.3 Postpartum Insertion". http://www.infoforhealth.org/pr/b6/b6chap3_3.shtml#top.
- ^ a b IUDs—An Update. "Chapter 3.4 Postabortion Insertion". http://www.infoforhealth.org/pr/b6/b6chap3_4.shtml#top.
- ^ Van Houdenhoven K, van Kaam K, van Grootheest A, Salemans T, Dunselman G (2006). "Uterine perforation in women using a levonorgestrel-releasing intrauterine system" (PDF). Contraception 73 (3): 257–60. doi:10.1016/j.contraception.2005.08.013. PMID 16472566. http://www.lareb.nl/documents/contraception2006_2210.pdf. Retrieved 2006-07-29.
- ^ "The influence of hormones and HIV genital transmission in women". Update Natl Minor AIDS Counc: 9–11. 1997. PMID 11367434.
- ^ "Progesterone-HIV link questioned by new studies". AIDS Alert 11 (7): 76–8. 1996. PMID 11363549.
"Progesterone and STDs: selected studies". Network 16 (4): 20–1. 1996. PMID 12291589.
Kiddugavu M, Makumbi F, Wawer M, Serwadda D, Sewankambo N, Wabwire-Mangen F, Lutalo T, Meehan M, Gray R (2003). "Hormonal contraceptive use and HIV-1 infection in a population-based cohort in Rakai, Uganda". AIDS 17 (2): 233–40. doi:10.1097/00002030-200301240-00014. PMID 12545084. - ^ McCarthy L (2006). "Levonorgestrel-Releasing Intrauterine System (Mirena) for Contraception". Am Fam Physician 73 (10): 1799–. http://www.aafp.org/afp/20060515/steps.html. Retrieved 2007-05-04.
- ^ Rönnerdag M, Odlind V (1999). "Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use". Acta Obstet Gynecol Scand 78 (8): 716–21. doi:10.1034/j.1600-0412.1999.780810.x. PMID 10468065.
- ^ FDA Medical Review p.6
- ^ Truitt S, Fraser A, Grimes D, Gallo M, Schulz K (2003). Lopez, Laureen M. ed. "Combined hormonal versus nonhormonal versus progestin-only contraception in lactation". Cochrane Database Syst Rev (2): CD003988. doi:10.1002/14651858.CD003988. PMID 12804497.
- ^ FDA Medical Review p.37
- ^ MIRENA® Data Sheet, Bayer NZ, 11 December 2009 http://www.bayerresources.com.au/resources/uploads/DataSheet/file9503.pdf Retrieved 2011-02-10
- ^ Schiappacasse V, Díaz S, Zepeda A, Alvarado R, Herreros C (2002). "Health and growth of infants breastfed by Norplant contraceptive implants users: a six-year follow-up study". Contraception 66 (1): 57–65. doi:10.1016/S0010-7824(02)00319-0. PMID 12169382.
- ^ (PDF) Medical Eligibility Criteria for Contraceptive Use. Third Edition. World Health Organization. 2004. pp. 101,113. http://www.who.int/reproductive-health/publications/mec/mec.pdf. Retrieved 2006-08-16.p.101, 113
- ^ FDA CDER (2000). "Mirena Pharmacology Review" (PDF). FDA. http://www.fda.gov/cder/foi/nda/2000/21-225.pdf_Mirena_Pharmr.pdf. Retrieved 2006-09-05.[dead link]
- ^ Hormonal Contraceptives, Progestogens Only. International Agency for Research on Cancer. 1999. http://www.inchem.org/documents/iarc/vol72/vol72-2.html. Retrieved 2006-10-08.
- ^ IUDs-An Update. Chapter 2.8: Intrauterine Pregnancy.
- ^ FDA Medical Review p. 5,41
- ^ FDA Medical Review pp. 3-4
- ^ Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit (2004). "FFPRHC Guidance (April 2004). The levonorgestrel-releasing intrauterine system (LNG-IUS) in contraception and reproductive health" (PDF). J Fam Plann Reprod Health Care 30 (2): 99–109. doi:10.1783/147118904322995474. PMID 15086994. http://www.ffprhc.org.uk/admin/uploads/IUSfinal.pdf.
- ^ Bahamondes L, Espejo-Arce X, Hidalgo MM, Hidalgo-Regina C, Teatin-Juliato C, Petta CA (2006). "A cross-sectional study of the forearm bone density of long-term users of levonorgestrel-releasing intrauterine system". Hum Reprod 21 (5): 1316–9. doi:10.1093/humrep/dei457. PMID 16373404.
- ^ a b IUDs—An Update. Chapter 2: Types of IUDs.
- ^ "Birth Control Options: The Progestasert Intrauterine Device (IUD)". Wyoming Health Council. 2004. http://wyhc.org/birth_control_options/Progestasert-IUD.php. Retrieved 2006-07-16.
- ^ FDA Medical Review p.10
- ^ http://www.wildemeersch.com/en/about
External links
- "IUDs—An Update". Population Information Program, the Johns Hopkins School of Public Health XXIII (5). December 1995. http://www.infoforhealth.org/pr/b6edsum.shtml.
- FDA (2000). "Medical review" (PDF scanned image). http://www.fda.gov/cder/foi/nda/2000/21-225.pdf_Mirena_Medr.pdf. - on Berlex Laboratories' Mirena application
- Physician Fact Sheet on Mirena
- = Mirena drug descritption/side effects
Birth control methods (G02B, G03A) Comparison Behavioral Avoiding vaginal intercourse: Abstinence • Anal sex • Masturbation • Non-penetrative sex • Oral sex
Including vaginal intercourse: Breastfeeding infertility (LAM) • Calendar-based methods (rhythm, etc.) • Fertility awareness • WithdrawalBarrier or
spermicidalHormonal
(formulations)Progestogen-onlyAnti-estrogen Ormeloxifene (Centchroman)Post-intercourse Emergency contraception (pills or copper IUD) (Yuzpe regimen, Ulipristal acetate)Intrauterine device IUD with copper (Paragard) • IUD with progestogen (Mirena)Abortion Sterilization Categories:- Hormonal contraception
- Intrauterine contraception
Wikimedia Foundation. 2010.