- Metal amides
-
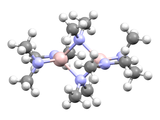
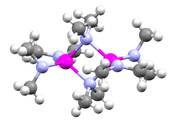
Metal amides are a class of coordination compounds composed of a metal center with amide ligands of the form NR2. Amide ligands have two electron pairs available for bonding. In principle, they can be terminal or bridging. In these two examples, the dimethylamido ligands are both bridging and terminal:
In practice, bulky amide ligands have a lesser tendency to bridge. Amide ligands may participate in metal-ligand π-bonding giving a complex with the metal center being co-planar with the nitrogen and substituents. Metal bis(trimethylsilyl)amides form a significant subcategory of metal amide compounds. These compounds tend to be discrete and soluble in organic solvent.
Alkali metal amides
Main articles: lithium amide, sodium amide, and potassium amideLithium amides are the most important amides, as they are readily prepared from n-butyllithium and the appropriate amine, and they are more stable and soluble than the other alkali metal analogs. Potassium amides are prepared by transmetallation of lithium amides with potassium t-butoxide (see also Schlosser base) or by reaction of the amine with potassium, potassium hydride, n-butylpotassium, or benzylpotassium.[3]
The alkali metal amides, MNH2 (M = Li, Na, K) are commercially available. Sodium amide (also known as sodamide) is synthesized from sodium metal and ammonia with ferric nitrate catalyst.[4][5] The sodium compound is white, but the presence of metallic iron turns the commercial material gray.
- 2 Na + 2 NH3 → 2 NaNH2 + H2
Lithium diisopropylamide is a popular non-nucleophilic base used in organic synthesis. Unlike many other bases, the steric bulk prevents this base from acting as a nucleophile. It is commercially available, usually as a solution in hexane. It may be readily prepared from n-butyllithium and diisopropylamine.
The amide ion is isoelectronic to the water molecule.
Transition metal complexes
Early transition metal amides may be prepared by treating anhydrous metal choride with alkali amide reagents, or with two equivalents of amine, the second equivalent acting as a base:[6]
- MCln + n LiNR2 → M(NR2)n + n LiCl
- MCln + 2n HNR2 → M(NR2)n + n HNR2·HCl
Late transition metal amide complexes may be prepared by:[6]
- treating a halide complex with an alkali amide
- treating an alkoxide complex with an amine
- deprotonation of a coordinated amine
- oxidative addition of an amine
References
- ^ Ouzounis, K.; Riffel, H.; Hess, H.; Kohler, U.; Weidlein, J. (1983). "Dimethylaminoalane, H3−nAl[N(CH3)2]n, n = 1, 2, 3 Kristallstrukturen und Molekülspektren". Zeitschrift für anorganische und allgemeine Chemie 504 (9): 67–76. doi:10.1002/zaac.19835040909.
- ^ Waggoner, K.M.; Olmstead, M.M.; Power, P.P. (1990). "Structural and spectroscopic characterization of the compounds [Al(NMe2)3]2, [Ga(NMe2)3]2, [(Me2N)2Al{μ-N(H)1-Ad}]2 (1-Ad = 1-adamantanyl) and [{Me(μ-NPh2)Al}2NPh(μ-C6H4)]". Polyhedron 9 (2–3): 257–263. doi:10.1016/S0277-5387(00)80578-1.
- ^ Michael Lappert, Andrey Protchenko, Philip Power, Alexandra Seeber (2009). "2. Alkali Metal Amides". Metal Amide Chemistry. John Wiley & Sons. ISBN 9780470740378.
- ^ Bergstrom, F. W. (1955), "Sodium Amide", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0778; Coll. Vol. 3: 778
- ^ Greenlee, K. W.; Henne, A. L.; Fernelius, W. Conard (1946). "Sodium Amide". Inorg. Synth.. Inorganic Syntheses 2: 128–135. doi:10.1002/9780470132333.ch38. ISBN 9780470132333.
- ^ a b John F. Hartwig (2009). "4. Covalent (X-Type) Ligands Bound Through Metal-Heteroatom Bonds". Organotransition Metal Chemistry: From Bonding to Catalysis. University Science Books. ISBN 189138953X.
Categories:- Coordination chemistry
- Metal amides
Wikimedia Foundation. 2010.