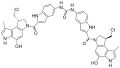
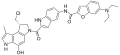
- Duocarmycin
-
The duocarmycins are a series of related natural products first isolated from Streptomyces bacteria in 1988.[1][2] They were found to have potent antitumor properties.[3]
Through the work of Dale L. Boger and others, the pharmacophore and mechanism of action were elucidated. The duocarmycins bind to the minor groove of DNA and alkylate the nucleobase adenine at the N3 position.[4][5] This research led to synthetic analogs including adozelesin, bizelesin, and carzelesin which progressed into clinical trials for the treatment of cancer.
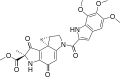
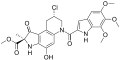
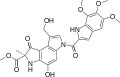
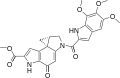
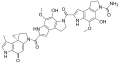
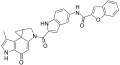
Duocarmycins
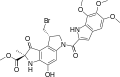
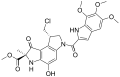
Clinical candidates
References
- ^ Yasuzawa, T; Iida, T; Muroi, K; Ichimura, M; Takahashi, K; Sano, H (1988). "Structures of duocarmycins, novel antitumor antibiotics produced by Streptomyces sp". Chemical & pharmaceutical bulletin 36 (9): 3728–31. PMID 3255306.
- ^ Takahashi, I; Takahashi, K; Ichimura, M; Morimoto, M; Asano, K; Kawamoto, I; Tomita, F; Nakano, H (1988). "Duocarmycin A, a new antitumor antibiotic from Streptomyces". The Journal of antibiotics 41 (12): 1915–7. PMID 3209484.
- ^ Boger, Dale L. (1991). "Duocarmycins: a new class of sequence selective DNA minor groove alkylating agents". Chemtracts: Organic Chemistry 4 (5): 329–349.
- ^ Dale L. Boger (1993). "Design, synthesis, and evaluation of DNA minor groove binding agents". Pure & Appl. Chern. 65 (6): 1123–1132. doi:10.1351/pac199365061123.
- ^ Dale L. Boger and Douglas S. Johnson (1995). "CC-1065 and the duocarmycins: Unraveling the keys to a new class of naturally derived DNA alkylating agents". Proc. Natl. Acad. Sci. USA 92 (9): 3642–3649. doi:10.1073/pnas.92.9.3642. PMC 42018. PMID 7731958. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=42018.
Categories:- Experimental cancer drugs
- Alkylating agents
- Alkaloids
Wikimedia Foundation. 2010.