- Digallic acid
-
Digallic acid 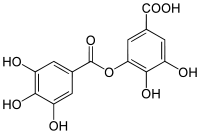
meta-depside bond digalloyl ester3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyl)oxybenzoic acidOther namesDigallate
3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyloxy)benzoate
m-digallic acid
Digalloyl esterIdentifiers CAS number 536-08-3 PubChem 341 Jmol-3D images Image 1 - C1=C(C=C(C(=C1O)O)O)C(=O)OC2=CC(=CC(=C2O)O)C(=O)[O-]
Properties Molecular formula C14H10O9 Molar mass 322.22 g/mol Exact mass 322.032481 u  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Digallic acid is a polyphenolic compound found in Pistacia lentiscus.[1] Digallic acid is also present in the molecule of tannic acid.[2] Digalloyl esters involve either -meta or -para depside bonds.[3]
Tannase is an enzyme that uses digallate to produce gallic acid.
References
- ^ Study of genotoxic, antigenotoxic and antioxidant activities of the digallic acid isolated from Pistacia lentiscus fruits. Wissem Bhouri, Safa Derbel, Ines Skandrani, Jihed Boubaker, Ines Bouhlel, Mohamed B. Sghaier, Soumaya Kilani, Anne M. Mariotte, Marie G. Dijoux-Franca, Kamel Ghedira and Leila Chekir-Ghedir, Toxicology in Vitro, Volume 24, Issue 2, March 2010, pp. 509-515, doi:10.1016/j.tiv.2009.06.024
- ^ Analysis of gallic, digallic and trigallic acids in tannic acids by high-performance liquid chromatography. P. Delahaye and M. Verzele, Journal of Chromatography A, Volume 265, 1983, pp. 363-367, doi:10.1016/S0021-9673(01)96734-2
- ^ Tannin chemistry, by Ann E. Hagerman
Aglycones Digallic acid | Gallic acid | Vanillic acidExamples Burkinabin A | B | C | Galloyl glucose | Digalloyl glucose | Trigalloyl glucose: 1,2,6-Trigalloyl glucose / 1,3,6,-Trigalloyl glucose (Corilagin) | Tetragalloyl glucose: 1,2,3,6-tetragalloylglucose | Pentagalloyl glucose: 1,2,3,4,6-pentagalloyl-glucose and 6-digalloyl-1,2,3-trigalloyl-glucose | Hexagalloyl glucose | Heptagalloyl glucose | Octagalloyl glucose | Nonagalloyl glucose | Decagalloyl glucose | 1,3,4-Tri-O-galloylquinic acid | 3,4,5-Tri-O-galloylquinic acid | 3,5-di-O-galloyl-shikimic acid | 3,4,5-tri-O-galloylshikimic acid | 1,2,6-trigalloyl alloside | 1,3,6-trigalloyl alloside | 1,2,3,6-tetragalloyl allosideThis article about a natural phenol is a stub. You can help Wikipedia by expanding it.
