- Rodiasine
-
Rodiasine 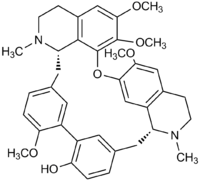
Identifiers CAS number 6391-64-6 PubChem 442345 ChemSpider 390797 
ChEBI CHEBI:8886 
Jmol-3D images Image 1 - O(c1ccc5cc1c2c(O)ccc(c2)C[C@H]7N(CCc6cc(OC)c(Oc3c4c(cc(OC)c3OC)CCN(C)[C@H]4C5)cc67)C)C
- InChI=1S/C38H42N2O6/c1-39-13-11-24-19-33(43-4)34-21-26(24)29(39)17-22-7-9-31(41)27(15-22)28-16-23(8-10-32(28)42-3)18-30-36-25(12-14-40(30)2)20-35(44-5)37(45-6)38(36)46-34/h7-10,15-16,19-21,29-30,41H,11-14,17-18H2,1-6H3/t29-,30+/m1/s1

Key: HIQZXOFBXJICTD-IHLOFXLRSA-N
InChI=1/C38H42N2O6/c1-39-13-11-24-19-33(43-4)34-21-26(24)29(39)17-22-7-9-31(41)27(15-22)28-16-23(8-10-32(28)42-3)18-30-36-25(12-14-40(30)2)20-35(44-5)37(45-6)38(36)46-34/h7-10,15-16,19-21,29-30,41H,11-14,17-18H2,1-6H3/t29-,30+/m1/s1
Key: HIQZXOFBXJICTD-IHLOFXLRBV
Properties Molecular formula C38H42N2O6 Molar mass 622.75 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Rodiasine is an cyclic bisbenzylisoquinoline alkaloid that was first isolated from the South American greenheart tree Chlorocardium rodiei.[1] The synthesis of O-demethylrodiasine (antioquine) and its derivatives, and the possible application of these compounds as anti-cancer, calcium channel blockers, and anti-parasitic drugs has been described.[2][3]
References
- ^ Grundon, M.F.; McGarvey, J.E.B. (1966). "Alkaloids from greenheart. Part III. The structure of rodiasine. Mass spectra of bisbenzylisoquinoline alkaloids". Journal of the Chemical Society C: Organic 1966: 1082–1084. doi:10.1039/J39660001082.
- ^ Bentley, K. W. (1996). "β-Phenylethylamines and the isoquinoline alkaloids". Natural Product Reports 13 (2): 127–150. doi:10.1039/NP9961300127. http://www.rsc.org/ejarchive/NP/1996/NP9961300127.pdf.
- ^ D'Ocon MP, Candenas ML, Anselmi E, Zafra-Polo MC, Cortes D (1989). "Antioquine: a new bisbenzylisoquinoleine alkaloid with calcium antagonist activity". Arch Int Pharmacodyn Ther 297: 205–16. PMID 2730236.

This article about an organic compound is a stub. You can help Wikipedia by expanding it.
