- Convergent synthesis
-
In chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multi-step chemical synthesis, most often in organic synthesis. In linear synthesis the overall yield quickly drops with each reaction step:
- A → B → C → D
Suppose the yield is 50% for each reaction, the overall yield of D is only 12.5% from A.
In a convergent synthesis
- A → B (50%)
- C → D (50%)
- B + D → E (25%)
the overall yield of E (25%) looks much better. Convergent synthesis is applied in the synthesis of complex molecules (see total synthesis) and involve fragment coupling and independent synthesis.
Examples:
- Convergent synthesis is encountered in dendrimer synthesis [1] where branches (with the number of generations preset) are connected to the central core
- Proteins of up to 300 amino acids are produced by a convergent approach using chemical ligation.
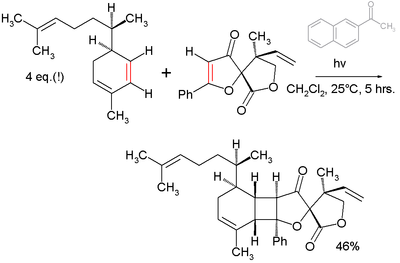
- An example of its use in total synthesis is the final step (photochemical [2+2]cycloaddition) towards the compound Biyouyanagin A [2]:
See also
References
- ^ Convergent Synthesis of Internally Branched PAMAM Dendrimers Michael Pittelkow, Jrn B. Christensen Org. Lett., 7 (7), 1295 -1298, 2005
- ^ Total Synthesis and Revised Structure of Biyouyanagin A K. C. Nicolaou, David Sarlah, and David M. Shaw Angew. Chem. Int. Ed. 2007, 46, 4708 –4711 doi:10.1002/anie.200701552
Categories:
Wikimedia Foundation. 2010.