- TonB-dependent receptors
Pfam_box
Symbol = TonB_dep_Rec
Name = TonB dependent receptor
width =250
caption =
Pfam= PF00593
InterPro= IPR000531
SMART=
PROSITE = PDOC00354
SCOP = 2fcp
TCDB = 1.B.14
OPM family= 89
OPM protein= 1qfg
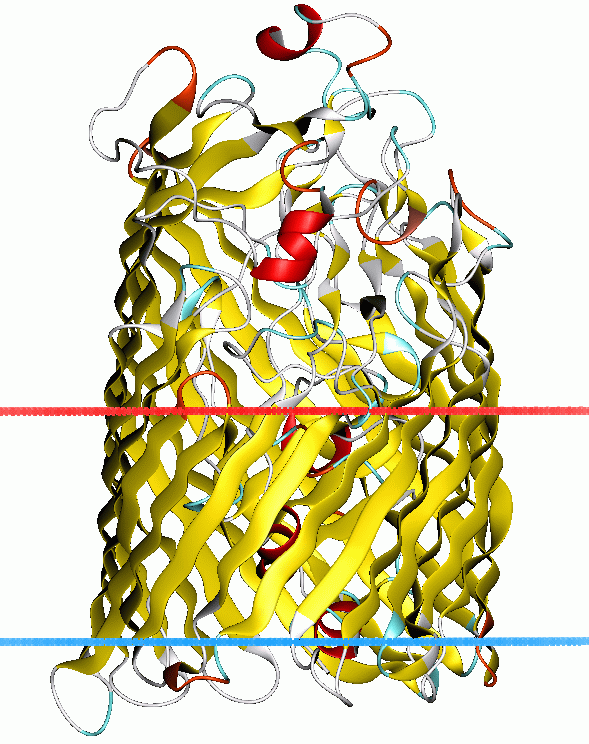
PDB=PDB3|1fepA:465-746 PDB3|1nqfA:390-614 PDB3|1ujwA:390-614PDB3|1nqhA:390-614 PDB3|1nqeA:390-614 PDB3|1nqgA:390-614PDB3|1qfgA:510-747 PDB3|1by5A:510-747 PDB3|1qkcA:510-747PDB3|1by3A:510-747 PDB3|2fcpA:510-747 PDB3|1fcpA:510-747PDB3|1qjqA:510-747 PDB3|1qffA:510-747 PDB3|1fi1A:510-747PDB3|1xkhA:570-815 PDB3|1xkwA:478-720 PDB3|1kmoA:525-774PDB3|1kmpA:525-774 PDB3|1po3B:525-774 PDB3|1pnzA:525-774PDB3|1po0A:525-774TonB-dependent receptors is a family of beta-barrel proteins from the outer membrane of
Gram-negative bacteria . The TonB complex senses signals from outside the bacterial cell and transmits them via two membranes into the cytoplasm, leading to transcriptional activation of targetgene s.In "
Escherichia coli " the TonB protein interacts with outer membrane receptor proteins that carry out high-affinity binding and energy-dependent uptake of specific substrates into the periplasmic spacecite journal |author=Kadner RJ, Chimento DP, Wiener MC |title=The Escherichia coli outer membrane cobalamin transporter BtuB: structural analysis of calcium and substrate binding, and identification of orthologous transporters by sequence/structure conservation |journal=J. Mol. Biol. |volume=332 |issue=5 |pages=999–1014 |year=2003 |pmid=14499604 |doi=10.1016/j.jmb.2003.07.005] . These substrates are either poorly permeable through the porin channels or are encountered at very low concentrations. In the absence of TonB, these receptors bind their substrates but do not carry out active transport. TonB-dependent regulatory systems consist of six protein protein components cite journal |author=Koebnik R |title=TonB-dependent trans-envelope signalling: the exception or the rule? |journal=Trends Microbiol. |volume=13 |issue=8 |pages=- |year=2005 |pmid=15993072 |doi=10.1016/j.tim.2005.06.005] .The proteins that are currently known or presumed to interact with TonB include BtuBcite journal |author=Kadner RJ, Chimento DP, Wiener MC, Mohanty AK |title=Substrate-induced transmembrane signaling in the cobalamin transporter BtuB |journal=Nat. Struct. Biol. |volume=10 |issue=5 |pages=394–401 |year=2003 |pmid=12652322 |doi=10.1038/nsb914] , CirA, FatA, FcuT, FecAcite journal |author=Deisenhofer J, Smith BS, Esser L, Chakraborty R, van der Helm D, Ferguson AD |title=Structural basis of gating by the outer membrane transporter FecA |journal=Science |volume=295 |issue=5560 |pages=1715–1719 |year=2002 |pmid=11872840 |doi=10.1126/science.1067313] , FhuAcite journal |author=Moras D, Rosenbusch JP, Mitschler A, Rees B, Locher KP, Koebnik R, Moulinier L |title=Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes |journal=Cell |volume=95 |issue=6 |pages=771–778 |year=1998 |pmid=9865695 |doi=10.1016/S0092-8674(00)81700-6] , FhuE, FepAcite journal |author=Deisenhofer J, Xia D, Buchanan SK, Smith BS, Venkatramani L, Esser L, Palnitkar M, Chakraborty R, van der Helm D |title=Crystal structure of the outer membrane active transporter FepA from Escherichia coli |journal=Nat. Struct. Biol. |volume=6 |issue=1 |pages=56–63 |year=1999 |pmid=9886293 |doi=10.1038/4931] , FptA, HemR, IrgA, IutA, PfeA, PupA and Tbp1. The TonB protein also interacts with some colicins. Most of these proteins contain a short conserved region at their N-terminuscite journal |author=Klebba PE |title=Three paradoxes of ferric enterobactin uptake |journal=Front. Biosci. |volume=8 |issue= |pages=- |year=2003 |pmid=12957833 |doi=10.2741/1233] .
TonB-dependent receptors include a plug domain which acts as the channel gate, blocking the pore until the channel is bound by ligand. At this point it under goes conformational changes opens the channel.
References
Wikimedia Foundation. 2010.
