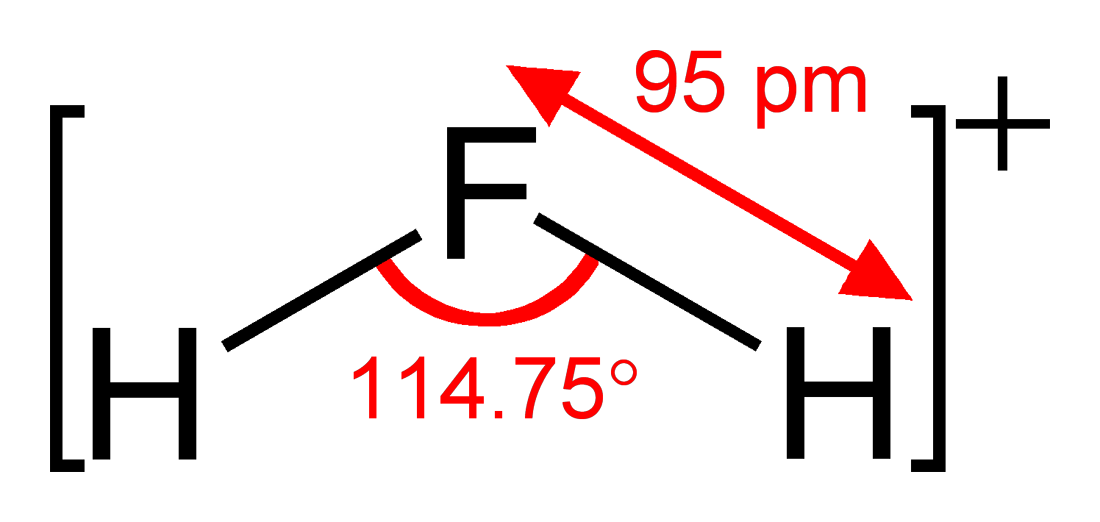
- Fluoronium
thumb|right|200px|The estimated [">Citation
last =Diercksen
first =G. H. F.
last2 =von Niessen
first2 =W.
last3 =Kraemer
first3 =W. P.
title =SCF LCGO MO studies on the fluoronium ion FH2+ and its hydrogen bonding interaction with hydrogen fluoride FH
journal =Theoretical Chemistry Accounts: Theory, Computation, and Modeling (Theoretica Chimica Acta)
volume =31
issue =3
pages =205-214
date =September
year =1973
url = http://www.springerlink.com/content/v517wg14w47676r4/
doi =10.1007/BF00526510
id = ] structure and dimensions of H2F+The fluoronium
cation , H2F+, is apolyatomic ion formed byprotonation or self-ionic dissociation ofhydrogen fluoride ::HF + H+ → H2F+
or
:3HF unicode|⇌ H2F+ + HF2−
Unlike for
halonium ion s based onchlorine ,bromine oriodine (chloronium , H2Cl+,bromonium , H2Br+,iodonium , H2I+), hydrocarbyl derivatives of fluoronium, i.e. R2F+, remain unknown.References
Wikimedia Foundation. 2010.