- Biochip
The development of biochips is a major thrust of the rapidly growing
biotechnology industry, which encompasses a very diverse range ofresearch efforts includinggenomics ,proteomics ,computational biology , andpharmaceuticals , among other activities. Advances inthese areas are giving scientists new methods for unraveling the complexbiochemical processes occurring inside cells, with the larger goal ofunderstanding and treating human diseases. At the same time, thesemiconductor industry has been steadily perfecting the science ofmicrominiaturization. The merging of these two fields in recent years hasenabled biotechnologists to begin packing their traditionally bulkysensing tools into smaller and smaller spaces, onto so-called biochips. Thesechips are essentially miniaturized laboratories that can perform hundreds orthousands of simultaneous biochemical reactions. Biochips enable researchersto quickly screen large numbers of biological analytes for a variety ofpurposes, from disease diagnosis to detection of bioterrorism agents.Definition
A biochip is a collection of miniaturized test sites (microarrays) arranged on a solid substrate that permits many tests to be performed at the same time in order to achieve higher output and speed.
History
The development of biochips has a long history, starting with early work onthe underlying
sensor technology. One of the first portable, chemistry-basedsensors was the glass pH electrode, invented in 1922 byHughes (Hughes, 1922). Measurement ofpH was accomplished bydetecting the potential difference developed across a thin glass membraneselective to the permeation of hydrogen ions; this selectivity was achievedby exchanges between H+ and SiO sites in the glass. The basic concept ofusing exchange sites to create permselective membranes was used to developother ion sensors in subsequent years. For example, a K+ sensor wasproduced by incorporatingvalinomycin into a thin membrane (Schultz, 1996).Over thirty years elapsed before the first truebiosensor ("i.e." asensor utilizing biological molecules) emerged. In 1956, Leland Clarkpublished a paper on an oxygen sensing electrode (Clark, 1956_41).This device became the basis for aglucose sensor developed in 1962 by Clarkand colleague Lyons which utilizedglucose oxidase molecules embedded in adialysis membrane (Clark, 1962). Theenzyme functioned in thepresence of glucose to decrease the amount of oxygen available to the oxygenelectrode, thereby relating oxygen levels to glucose concentration. This andsimilar biosensors became known as enzyme electrodes, and are still in usetoday.In 1953,
Watson and Crick announced their discovery of the now familiardouble helix structure ofDNA molecules and set the stage forgenetics research that continues to the present day (Nelson, 2000). The developmentofsequencing techniques in 1977 by Gilbert (Maxam, 1977) and
Sanger (Sanger, 1977) (working separately) enabled researchers todirectly read the genetic codes that provide instructions for
protein synthesis. This research showed how hybridization of complementary singleoligonucleotide strands could be used as a basis for DNA sensing. Twoadditional developments enabled the technology used in modern DNA-basedbiosensors. First, in 1983Kary Mullis invented thepolymerase chain reaction (PCR) technique (Nelson, 2000), a method for amplifying DNA concentrations.This discovery made possible the detection of extremely small quantities ofDNA in samples. Second, in 1986 Hood and coworkers devised a method to labelDNA molecules withfluorescent tag s instead ofradiolabels (Smith, 1986), thus enabling hybridization experiments tobe observed optically.The rapid technological advances of the
biochemistry andsemiconductor fieldsin the 1980s led to the large scale development of biochips in the 1990s.At this time, it became clear that biochips were largely a "platform"technology which consisted of several separate, yet integrated components.Figure 1 shows the makeup of a typical biochip platform.The actual sensing component (or "chip") is just one piece of a completeanalysis system. Transduction must be done to translate the actual sensingevent (DNA binding, oxidation/reduction, "etc.") into a formatunderstandable by a computer (voltage , light intensity, mass, "etc."),which then enables additional analysis and processing to produce a final,human-readable output. The multiple technologies needed to make a successfulbiochip — from sensing chemistry, tomicroarray ing, to signal processing —require a true multidisciplinary approach, making the barrier to entry steep.One of the first commercial biochips was introduced byAffymetrix . Their"GeneChip" products contain thousands of individual DNA sensors for use insensing defects, or single nucleotide polymorphisms (SNPs), in genes such asp53 (a tumor suppressor) andBRCA1 andBRCA2 (related to breastcancer) (Cheng, 2001). The chips are produced usingmicrolithography techniques traditionally used to fabricateintegrated circuits (see below).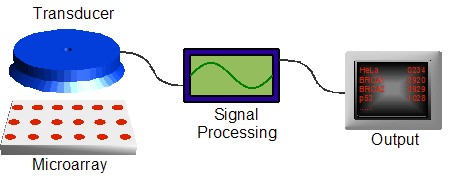
frame|thumb right|300 px|Figure 1. Biochips are a platform that require, inaddition to microarray technology, transduction and signal processingtechnologies to output the results of sensing experiments.Today, a large variety of biochip technologies are either in development orbeing commercialized. Numerous advancements continue to be made in sensingresearch that enable new platforms to be developed for new applications.Cancer diagnosis through DNA typing is just one market opportunity. A varietyof industries currently desire the ability to simultaneously screen for awide range of chemical and biological agents, with purposes ranging fromtesting public water systems for disease agents to screening airline cargofor explosives. Pharmaceutical companies wish to combinatorially screen drugcandidates against target enzymes. To achieve these ends,
DNA ,RNA ,proteins ,and even living cells are being employed as sensing mediators on biochips.Numerous transduction methods can be employed includingsurface plasmon resonance ,fluorescence , andchemiluminescence . The particular sensing andtransduction techniques chosen depend on factors such as price, sensitivity,and reusability.Microarray fabrication
The microarray — the dense, two-dimensional grid of biosensors — is the critical component of a biochip platform. Typically, the sensors are deposited on a flat substrate, which may either be passive ("e.g." silicon or glass) or active, the latterconsisting of integrated electronics or micromechanical devices that perform or assist signal transduction.
Surface chemistry is used to covalently bind the sensor molecules to the substrate medium. The fabrication of microarrays is non-trivial and is a major economic and technological hurdle that mayultimately decide the success of future biochip platforms. The primary manufacturing challenge is the process of placing each sensor at a specific position (typically on aCartesian grid) on the substrate. Various means exist to achieve the placement, but typically robotic micro-pipetting (Schena, 1995) or micro-printing (MacBeath, 1999) systems are used to place tiny spots of sensor material on the chip surface. Because each sensor is unique, only a few spots can be placed at a time. The low-throughput nature of thisprocess results in high manufacturing costs.Fodor and colleagues developed a unique fabrication process (later used by
Affymetrix ) in which a series of microlithography steps is used to
combinatorially synthesize hundreds of thousands of unique, single-strandedDNA sensors on a substrate onenucleotide at atime (Fodor, 1991; Pease, 1994). One lithography step is needed per base type; thus, a totalof four steps is required per nucleotide level. Although this technique isvery powerful in that many sensors can be created simultaneously, it iscurrently only feasible for creating short DNA strands (15–25 nucleotides).Reliability and cost factors limit the number of photolithography steps thatcan be done. Furthermore, light-directed combinatorial synthesis techniquesare not currently possible for proteins or other sensing molecules.As noted above, most microarrays consist of a Cartesian grid of sensors. Thisapproach is used chiefly to map or "encode" the coordinate of each sensorto its function. Sensors in these arrays typically use a universal signalingtechnique ("e.g." fluorescence), thus making coordinates their onlyidentifying feature. These arrays must be made using a serial process("i.e." requiring multiple, sequential steps) to ensure that each sensoris placed at the correct position.
"Random" fabrication, in which the sensors are placed at arbitrarypositions on the chip, is an alternative to the serial method. The tedious and expensive positioning process isnot required, enabling the use of parallelized self-assembly techniques. Inthis approach, large batches of identical sensors can be produced; sensorsfrom each batch are then combined and assembled into an array. Anon-coordinate based encoding scheme must be used to identify each sensor. Asthe figure shows, such a design was first demonstrated (and latercommercialized by Illumina) using functionalized beads placed randomly in thewells of an etched
fiber optic cable (Steemers, 2000; Michael, 1998) Each bead was uniquelyencoded with a fluorescent signature. However, this encoding scheme islimited in the number of unique dye combinations that be can be used andsuccessfully differentiated.Protein biochip array and other microarray technologies
Microarray s are not limited toDNA analysis;protein microarray s,antibody microarray ,chemical compound microarray can also be produced using biochips. [http://www.randox.com Randox] Laboratories Ltd. launched Evidence, the first protein Biochip Array Technology analyzer in 2003. In protein Biochip Array Technology, the biochip replaces theELISA plate orcuvette as the reaction platform. The biochip is used to simultaneously analyze a panel of related tests in a single sample, producing apatient profile. The patient profile can be used in disease screening,diagnosis , monitoring disease progression or monitoring treatment. Performing multiple analyses simultaneously, described as multiplexing, allows a significant reduction in processing time and the amount of patient sample required. Biochip Array Technology is a novel application of a familiar methodology, using sandwich, competitive and antibody-captureimmunoassay s. The difference from conventional immunoassays is that the capture ligands are covalently attached to the surface of the biochip in an ordered array rather than in solution.In sandwich assays an enzyme-labelled antibody is used; in competitive assays an enzyme-labelled antigen is used. On antibody-antigen binding a
chemiluminescence reaction produces light. Detection is by acharge-coupled device (CCD) camera. The CCD camera is a sensitive and high-resolution sensor able to accurately detect and quantify very low levels of light. The test regions are located using a grid pattern then the chemiluminescence signals are analysed by imaging software to rapidly and simultaneously quantify the individual analytes.Details about other array technologies can be found in the following pages:
Antibody microarray andchemical compound microarray .See also
*
DNA microarray
*Protein array
*Chemical compound microarray
*Antibody microarray
*Tissue microarray
*Single nucleotide polymorphism
*Sequencing
*Lab-on-a-chip
* Planar Patch Clamp
*Nanosensors References
* W. S. Hughes, “The potential difference between glass and electrolytes in contact with water,” "J. Am. Chem. Soc." 44, pp. 2860–2866, 1922.
* J. S. Schultz and R. F. Taylor in "Handbook of Chemical and Biological Sensors", J. S. Schultz and R. F. Taylor, eds., ch. Introduction to Chemical and Biological Sensors, pp. 1–10, Institute of Physics Publishing, Philadelphia, 1996.
* L. C. Clark, Jr., “Monitor and control of blood tissue O2 tensions,” "Transactions of the American Society for Artificial Internal Organs" 2, pp. 41–84, 1956.
* L. C. Clark, Jr. and C. Lyons, “Electrode system for continuous monitoring in cardiovascular surgery,” "Annals of the New York Academy of Sciences" 148, pp. 133–153, 1962.
* D. L. Nelson and M. M. Cox, "Lehninger Principles of Biochemistry", Worth Publishers, New York, 2000.
* A. M. Maxam and W. Gilbert, “A new method for sequencing DNA,” "Proc. Nat. Acad. Sci." 74, pp. 560–564, 1977.
* F. Sanger, S. Nicklen, and A. R. Coulson, “DNA sequencing with chainterminating inhibitors,” "Proc. Nat. Acad. Sci." 74, pp. 5463–5467, 1977.
* L. M. Smith, J. Z. Sanders, R. J. Kaiser, P. Hughes, C. Dodd, C. R. Connell, C. Heiner, S. B. H. Kent, and L. E. Hood, “Fluorescence detection in automated DNA sequence analysis,” "Nature" 321, pp. 61–67, 1986.
* P. Fortina, D. Graves, C. Stoeckert, Jr., S. McKenzie, and S. Surrey in "Biochip Technology", J. Cheng and L. J. Kricka, eds., ch. Technology Options and Applications of DNA Microarrays, pp. 185–216, Harwood Academic Publishers, Philadelphia, 2001.
* M. Schena, D. Shalon, R. W. Davis, and P. O. Brown, “Quantitative monitoring of gene expression patterns with a complementary DNA microarray,” "Science" 270, pp. 467–470, 1995.
* G. MacBeath, A. N. Koehler, and S. L. Schreiber, “Printing small molecules as microarrays and detecting protein-ligand interactions en masse,” "J. Am. Chem. Soc." 121, pp. 7967–7968, 1999.
* S. P. Fodor, J. L. Read, M. C. Pirrung, L. Stryer, A. T. Lu, and D. Solas, “Light-directed, spatially addressable parallel chemical analysis,” "Science" 251, pp. 767–773, 1991.
* A. C. Pease, D. Solas, E. J. Sullivan, M. T. Cronin, C. P. Holmes, and S. P. Fodor, “Light-generated oligonucleotide arrays for rapid DNA sequence analysis,” "Proc. Natl. Acad. Sci." 91, pp. 5022–5026, 1994.
* F. J. Steemers, J. A. Ferguson, and D. R. Walt, “Screening unlabeled DNA targets with randomly-ordered fiber-optic gene arrays,” "Nature Biotechnology" 18, pp. 91–94, 2000.
* K. L. Michael, L. C. Taylor, S. L. Schultz, and D. R. Walt, “Randomly ordered addressable high-density optical sensor arrays,” "Analytical Chemistry" 70, pp. 1242–1248, 1998.
* K. L. Gunderson, S. Kruglyak, M. S. Graige, F. Garcia, B. G. Kermani, C. Zhao, D. Che, T. Dickinson, E. Wickham, J. Bierle, D. Doucet, M. Milewski, R. Yang, C. Siegmund, J. Haas, L. Zhou, A. Oliphant, J.-B. Fan, S. Barnard, and M. S. Chee, “Decoding randomly ordered DNA arrays,” "Genome Research" 14(5), pp. 870–877, 2004.
* C. Roberts, C. S. Chen, M. Mrksich, V. Martichonok, D. E. Ingber, and G. M. Whitesides, “Using mixed self-assembled monolayers presenting RGD and (EG)3OH groups to characterize long-term attachment of bovine capillary endothelial cells to surfaces,” "J. Am. Chem. Soc." 120, pp. 6548–6555, 1998.
*H. Schmeck, "Blazing the Genetic Trail." Bethesda, MD: Howard Hughes Medical Institute, 1991.http://www.accessexcellence.org/RC/AB/IE/Future_Of_Genetic_Research.html
*Interview of A. Caplan, "Should We or Shouldn't We?" http://web.reed.edu/reed_magazine/spring06/features/life_in_venice/should_we.html
*Vahid Bemanian, Frøydis D. Blystad, Live Bruseth, Gunn A. Hildrestrand, Lise Holden, Endre Kjærland, Pål Puntervoll, Hanne Ravneberg and Morten Ruud, "What is Bioethics?" Dec 1998.http://www.uib.no/People/mblpp/bioethics/Bioethics.html
*M. Burnham, R. Mitchell, " Bioethics — An Introduction" 1992.http://www.woodrow.org/teachers/bi/1992/bioethics_intro.html
*NBIAP NEWS REPORT, U.S. Department of Agriculture, "To Regulate or Not to Regulate" Forum: To Rationalize U.S. Biotech Regs. June 1994http://www.accessexcellence.org/RC/AB/IE/To_Regulate_or_Not.htmlBiochip definitionhttp://searchcio-midmarket.techtarget.com/sDefinition/0,,sid183_gci211664,00.html#
Wikimedia Foundation. 2010.
