- Constant flux calorimetry
-
A key part of Reaction Calorimetry is the ability to control temperature in the face of extreme thermal events. Once the temperature is able to be controlled measurement of a variety of parameters can allow an understanding of how much heat is being released of absorbed by a reaction.
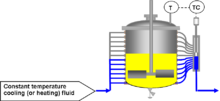
In essence COFLUX or Constant flux calorimetry is a highly developed temperature control mechanism which can be used to generate highly accurate calorimetry. It works by controlling the jacket area of a controlled lab reactor, while keeping the inlet temperature of the thermal fluid constant. This allows the temperature to be precisely controlled even under strongly exothermic or endothermic events as additional cooling is always available by simply increasing the area over which the heat is being exchanged.
This system is generally more accurate than heat balance calorimetry (on which it's based) as changes in the delta temperature (Tout - Tin) are magnified by keeping the fluid flow as low as possible.
Theory of COFLUX
One of the main advantages of COFLUX calorimetry is the ability to dynamically measure heat transfer coefficient (U). We know from the heat balance equation that:
- Q = mf.Cpf.Tin - Tout
We also know that from the heat flow equation that
- Q = U.A.LMTD
We can therefore rearrange this such that
- U = mf.Cpf.Tin - Tout /A.LMTD
This will allow us therefore to monitor U as a function of time.
Specific applications of COFLUX
- COFLUX temperature control
- COFLUX for Crystallisation
- COFLUX for scale up modelling
- COFLUX for calorimetry
See also

This article about analytical chemistry is a stub. You can help Wikipedia by expanding it.