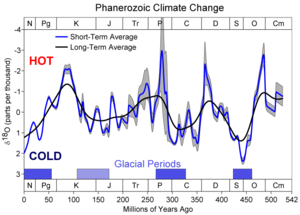
- Oxygen isotope ratio cycle
-
Oxygen isotope ratio cycles are cyclical variations in the ratio of the abundance of oxygen with an atomic mass of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite in ocean core samples. The ratio is linked to water temperature of ancient oceans, which in turn reflects ancient climates. Cycles in the ratio mirror climate changes in geologic history.
Contents
Isotopes of oxygen
Oxygen (chemical symbol O) has three naturally occurring isotopes: 16O, 17O, and 18O, where the 16, 17 and 18 refer to the atomic mass. The most abundant is 16O, with a small percentage of 18O and an even smaller percentage of 17O. Oxygen isotope analysis considers only the ratio of 18O to 16O present in a sample.
The calculated ratio of the masses of each present in the sample is then compared to a standard, which can yield information about the temperature at which the sample was formed - see Proxy (climate) for details.
Connection between isotopes and temperature/weather
18O is two neutrons heavier than 16O and causes the water molecule in which it occurs to be heavier by that amount. The addition of more energy is required to vaporize H218O than H216O, and H218O liberates more energy when it condenses. In addition, H216O tends to diffuse more rapidly.
Because H216O requires less energy to vaporize, and is more likely to diffuse to the liquid surface, the first water vapor formed during evaporation of liquid water is enriched in H216O, and the residual liquid is enriched in H218O. When water vapor condenses into liquid, H218O preferentially enters the liquid, while H216O is concentrated in the remaining vapor.
As an air mass moves from a warm region to a cold region, water vapor condenses and is removed as precipitation. The precipitation removes H218O, leaving progressively more H216O-rich water vapor. This distillation process causes precipitation to have lower 18O/16O as the temperature decreases. Additional factors can affect the efficiency of the distillation, such as the direct precipitation of ice crystals, rather than liquid water, at low temperatures.
Due to the intense precipitation that occurs in hurricanes, the H218O is exhausted relative to the H216O, resulting in relatively low 18O/16O ratios. The subsequent uptake of hurricane rainfall in trees, creates a record of the passing of hurricanes that can be used to create a historical record in the absence of human records.[1]
Connection between temperature and climate
The 18O/16O ratio provides a record of ancient water temperature. Water 10 to 15 degrees Celsius (18 to 27 degrees Fahrenheit) cooler than present represents glaciation. Precipitation and therefore glacial ice contain water with a low 18O content. Since large amounts of 16O water are being stored as glacial ice, the 18O content of oceanic water is high. Water up to 5 degrees Celsius (9 °F) warmer than today represents an interglacial, when the 18O content of oceanic water is lower. A plot of ancient water temperature over time indicates that climate has varied cyclically, with large cycles and harmonics, or smaller cycles, superimposed on the large ones. This technique has been especially valuable for identifying glacial maxima and minima in the Pleistocene.
Connection between calcite and water
Limestone is deposited from the calcite shells of microorganisms. Calcite, or calcium carbonate, chemical formula CaCO3, is formed from water, H2O, and carbon dioxide, CO2, dissolved in the water. The carbon dioxide provides two of the oxygen atoms in the calcite. The calcium must rob the third from the water. The isotope ratio in the calcite is therefore the same, after compensation, as the ratio in the water from which the microorganisms of a given layer extracted the material of the shell. The microorganism most frequently referenced is foraminifera.
The sentence "The isotope ratio in the calcite is therefore the same, ..." is inexact: "proportionnal" would be more accurate (actually 1/3 of Oxygen present in calcite CaCO3; That is, without further chemistry details)
References
- ^ Miller, Dana L.; Mora, Claudia I.; Grissino-Mayer, Henri D.; Mock, Cary J.; Uhle, Maria E.; Sharp, Zachary (July 31, 2006/September 19, 2006). "Tree-ring isotope records of tropical cyclone activity". Proceedings of the National Academy of Sciences, 2006 - National Acad Sciences. 103 no. 39. National Acad Sciences. pp. 14294–14297. doi:10.1073/pnas.0606549103. http://www.pnas.org/content/103/39/14294.full. Retrieved 2009-11-11.
- Encyclopædia Britannica under Climate and Weather, Pleistocene Climatic Change
- Harmon Craig. 1961. "Isotopic variations in meteoric waters". Science. 133. pp 1702
- S. Epstein, T. Mayeda. 1953. "Variation of O18 content of waters from natural sources", Geochemica et Cosmochemica Acta. 4. pp 213
External links
Categories:
Wikimedia Foundation. 2010.