- Osmium borides
-
Osmium borides are compounds of osmium and boron. Their most remarkable property is potentially high hardness. It is thought that a combination of high electron density of osmium with the strength of boron-osmium covalent bonds will make osmium borides superhard materials. This has not been demonstrated yet. For example, OsB2 is hard (hardness comparable to that of sapphire), but not superhard.[1]
Synthesis
Osmium borides are produced in vacuum or inert atmosphere to prevent formation of osmium tetroxide, which is a hazardous compound. Synthesis occurs at high temperatures (~1000 °C) from a mixture of MgB2 and OsCl3.[1]
Structure
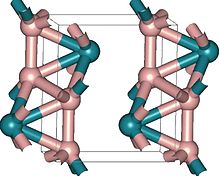
Three osmium borides are known: OsB1.1, Os2B3 and OsB2. The first two have hexagonal structure,[2] similar to that of rhenium diboride. Osmium diboride was first also sought as hexagonal,[3] but its structure was later reassigned to orthorhombic.[1][4]
References
- ^ a b c Cumberland, Robert W.; et al. (April 27, 2005). "Osmium Diboride, An Ultra-Incompressible, Hard Material". Journal of the American Chemical Society 127 (20): 7264. doi:10.1021/ja043806y. PMID 15898746.
- ^ M. Hebbache et al. (2006). "A new superhard material: Osmium diboride OsB2". Solid State Communications 139 (5): 227–231. doi:10.1016/j.ssc.2006.05.041.
- ^ Kempter, C. P.; Fries, R. J. (1961). "Crystallography of the Ru-B and Os-B Systems". The Journal of Chemical Physics 34 (6): 1994. doi:10.1063/1.1731807.
- ^ Roof, R. B.; Kempter, C. P. (1962). "New Orthorhombic Phase in the Ru-B and Os-B Systems". The Journal of Chemical Physics 37 (7): 1473. doi:10.1063/1.1733309.
Categories:- Osmium compounds
- Borides
- Superhard materials
Wikimedia Foundation. 2010.