- Wagner–Meerwein rearrangement
-
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.[1][2]
Several reviews have been published.[3][4][5][6][7]
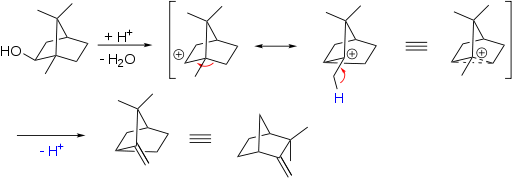
The rearrangement was first discovered in bicyclic terpenes for example the conversion of isoborneol to camphene [8]:
The story of the rearrangement reveals that many scientists were puzzled with this and related reactions and its close relationship to the discovery of carbocations as intermediates.[9]
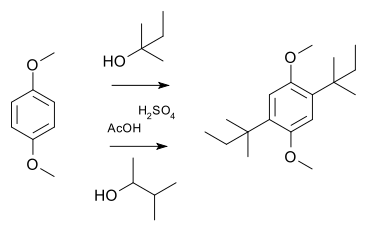
In a simple demonstration reaction of 1,4-dimethoxybenzene with either 2-methyl-2-butanol or 3-methyl-2-butanol in sulfuric acid and acetic acid yields the same disubstituted product,[10] the latter via a hydride shift of the cationic intermediate:
Currently, there are works relating to the use of skeletal rearrangement in the synthesis of bridged azaheterocycles. These data are summarized in [11]
The related Nametkin rearrangement named after Sergey Namyotkin involves the rearrangement of methyl groups in certain terpenes. In some cases the reaction type is also called a retropinacol rearrangement (see Pinacol rearrangement).
References
- ^ Wagner, G. J. Russ. Phys. Chem. Soc. 1899, 31, 690.
- ^ Hans Meerwein (1914). "Über den Reaktionsmechanismus der Umwandlung von Borneol in Camphen; [Dritte Mitteilung über Pinakolinumlagerungen.]". Justus Liebig's Annalen der Chemie 405: 129–175. doi:10.1002/jlac.19144050202.
- ^ Popp, F. D.; McEwen, W. E. Chem. Rev. 1958, 58, 375. (Review)
- ^ Cargill, R. L. et al. Accts. Chem. Res. 1974, 7, 106–113. (Review)
- ^ Olah, G. A. Accts. Chem. Res. 1976, 9, 41. (Review)
- ^ Hogeveen, H.; Van Krutchten, E. M. G. A. Top. Curr. Chem. 1979, 80, 89–124. (Review)
- ^ Hanson, J. R. Comp. Org. Syn. 1991, 3, 705–719. (Review)
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ^ Birladeanu, L. J. Chem. Ed. 2000, 77, 858–863.
- ^ Carbocation Rearrangement in an Electrophilic Aromatic Substitution Discovery Laboratory Victoria Polito, Christian S. Hamann and Ian J. Rhile J. Chem. Educ., 2010, 87 (9), pp 969–970 doi:10.1021/ed9000238
- ^ Aza-Heterocycles in Wagner–Meerwein Rearrangement: “Skeletal Wagner–Meerwein rearrangement of perhydro-3a,6;4,5-diepoxyisoindoles” Zubkov, F. I. ; Zaytsev, V. P.; Nikitina, E. V.; Khrustalev, V. N.; Gozun, S. V.; Boltukhina, E. V.; Varlamov, A. V. Tetrahedron 2011, 67, 9148-9163 doi:10.1016/j.tet.2011.09.099
See also
Categories:- Rearrangement reactions
- Name reactions
Wikimedia Foundation. 2010.