Pfitzner–Moffatt oxidation
- Pfitzner–Moffatt oxidation
-
The Pfitzner–Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction which describes the oxidation of primary and secondary alcohols by dimethyl sulfoxide (DMSO) activated with a carbodiimide, such as dicyclohexylcarbodiimide (DCC). The resulting alkoxysulfonium ylide rearranges to generate aldehydes and ketones, respectively.[1][2]
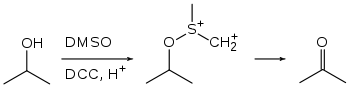
This reaction has been largely abandoned for the Swern oxidation, which gives higher yields with fewer side products. The Moffatt oxidation yields urea biproducts that are often difficult to remove.
Several reviews have been published.[3][4]
See also
References
- ^ Pfitzner, K. E.; Moffatt, J. G. (1963). "A New and Selective Oxidation of Alcohols". J. Am. Chem. Soc. 85: 3027. doi:10.1021/ja00902a036.
- ^ J. G. Moffatt, “Sulfoxide-Carbodiimide and Related Oxidations” in Oxidation vol. 2, R. L. Augustine, D. J. Trecker, Eds. (Dekker, New York, 1971) pp 1–64.
- ^ Tidwell, T. T. Org. React. 1990, 39, 297–572. (Review)
- ^ Lee, T. V. Comp. Org. Syn. 1991, 7, 291–303. (Review)
Categories:
- Organic redox reactions
- Name reactions
Wikimedia Foundation.
2010.
Look at other dictionaries:
Pfitzner-Moffatt oxidation — The Pfitzner Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction which describes the oxidation of primary and secondary alcohols by dimethyl sulfoxide (DMSO) activated with a carbodiimide, such as… … Wikipedia
Pfitzner-Moffatt-Oxidation — Die Pfitzner Moffatt Oxidation, oft auch einfach Moffat Oxidation, ist eine milde Oxidationsmethode, die primäre und sekundäre Alkohole mit N,N’ Dicyclohexylcarbodiimid (DCC) und Dimethylsulfoxid (DMSO) in die jeweiligen Aldehyde oder Ketone… … Deutsch Wikipedia
Pfitzner-Moffatt-Oxidation — Pfịtz|ner Mọf|fatt O|xi|da|ti|on [nach dem amer. Chemiker K. E. Pfitzner u. dem kanad. Chemiker J. G. Moffatt (*1930)]: Überführung von Alkoholen in Carbonylverb. durch Dehydrierung mittels Dimethylsulfoxid u. Dicyclohexylcarbodiimid … Universal-Lexikon
Moffatt — ist der Name folgender Personen: Ariane Moffatt (* 1979), franko kanadische Chansonnière Emma Moffatt (* 1984), australische Triathletin Hugh Moffatt (* 1948), US amerikanischer Country Sänger und Songwriter Jerry Moffatt (* 1963), englischer… … Deutsch Wikipedia
Moffatt — may refer to: Moffat (surname), spelling variant Moffatt, New Translation, 1926 translation of the Bible Moffatt oxidation, also known as Pfitzner Moffatt oxidation Moffatt Township, Michigan The Moffatts, Canadian band See also Moffat… … Wikipedia
Oxidation durch hypervalente Iod-Reagenzien — Die Oxidation durch hypervalente Iod Reagenzien umfasst in der organische Chemie die Oxidation von primären bzw. sekundären Alkoholen durch die Oxidationsmittel IBX (2 Iodoxybenzoesäure) und Dess Martin Periodinan. Vor allem die Dess Martin… … Deutsch Wikipedia
Swern oxidation — The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. [cite… … Wikipedia
Dess-Martin-Oxidation — Die Dess Martin Oxidation ist eine chemische Reaktion, die zur Oxidation von primären und sekundären Alkoholen zu Aldehyden bzw. Ketonen benutzt wird. Als Oxidationsmittel wird das Dess Martin Periodinan verwendet. Es handelt sich um eine sehr… … Deutsch Wikipedia
Alcohol oxidation — Mechanism of oxidation of primary alcohols to carboxylic acids via aldehydes and aldehyde hydrates Alcohol oxidation is an important organic reaction. Primary alcohols (R CH2 OH) can be oxidized either to aldehydes (R CHO) or to carboxylic acids… … Wikipedia
Corey-Kim oxidation — The Corey Kim oxidation is an oxidation reaction used to synthesise aldehydes and ketones from primary and secondary alcohols. [cite journal title = New and highly effective method for the oxidation of primary and secondary alcohols to carbonyl… … Wikipedia

