- Wulff-Dötz reaction
-
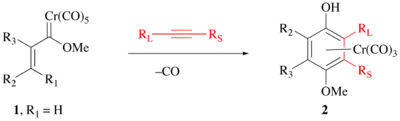
The Wulff-Dötz reaction (also known as the Dötz reaction or the benzannulation reaction of the Fischer carbene complexes) is the chemical reaction of an aromatic or vinylic alkoxy pentacarbonyl chromium carbene complex with an alkyne and carbon monoxide to give a Cr(CO)3-coordinated substituted phenol.[1][2][3] Several reviews have been published.[4][5][6] It is named after the German chemist Karl Heinz Dötz (b. 1943) and the American chemist William D. Wulff (b. 1949) at Michigan State University.[7] The reaction was first discovered by Karl Dötz and was extensively developed by his group and W. Wulff's group. They subsequently share the name of the reaction.
The position of the substituents is highly predictable with the largest alkyne substituent (RL) neighboring the phenol and the smallest alkyne substituent (RS) neighboring the methoxy group .[8][9] Hence, this reaction is more useful for terminal alkynes than internal alkynes.
The phenol can be liberated from the chromium complex by a mild oxidation, such as ceric ammonium nitrate or air oxidation.
Since this reaction can quickly generate complex phenolic compounds, the Wulff-Dötz reaction has been used most often in the synthesis of natural products, especially Vitamins E and K.[10][11]
Mechanism
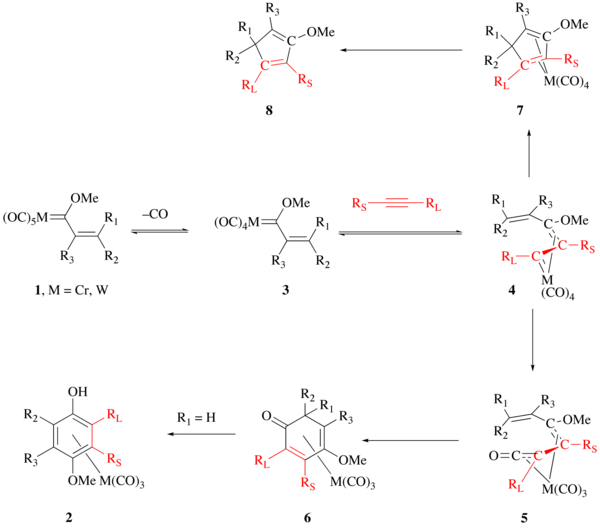
The exact details of the Wulff-Dötz reaction are still being discussed and argued. The currently accepted mechanism of this reaction is outlined below. The first step of the reaction involves the loss of carbon monoxide from the Fischer carbene complex 1 to give intermediate 3. The loss of CO is rate limiting making the investigation of this reaction mechanism difficult. The alkyne then coordinates to the metal center which has been calculated as a low-energy barrier process with a ΔE‡ of 2.0-4.0 kcal/mol (for terminal alkynes at B3LYP/SVP). The resulting alkyne metal coordinated complex immediately rearranges to intermediate 4 with activation barrier of 3-9 kcal/mol (for terminal acetylenes at B3LYP/SVP). The η1, η3-complex shown as 4 subsequently undergoes CO insertion to give the η4-vinylketene complex 5 which undergoes electrocyclization to give intermediate 6. When R1 is hydrogen, intermediate 6 is short lived and proceeds to the metal tricarbonyl arene complex 2. Without CO insertion, the reaction proceeds through 7 to the cyclopentadiene product 8.
References
- ^ Dötz, K. H. (1975). "Synthesis of the Naphthol Skeleton from Pentacarbonyl-[methoxy(phenyl)carbene]chromium (O) and Tolan". Angew. Chem. Int. Ed. Engl. 14: 644–645. doi:10.1002/anie.197506442.
- ^ Dötz, K. H.; Dietz, R.; von Imhof, A.; Lorenz, H.; Huttner, G. (1976). "Reaktionen von Komplexliganden, IV. Stereoselektive Synthese substituierter Naphthaline: Darstellung und Struktur eines Tricarbonyl(naphthalin)chrom(0)-Komplexes". Chem. Ber. 109: 2033. doi:10.1002/cber.19761090610.
- ^ Timko, J. M.; Yamashita, A. (1998), "Synthesis of 2-substituted naphthalenediol derivatives using chromium carbene complexes: 1-acetoxy-2-butyl-4-methoxynaphthalene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv9p0001; Coll. Vol. 9: 1
- ^ Waters, M. L.; Wulff, W. D. Org. React. 2008, 70, 121. (doi: 10.1002/0471264180.or070.02)
- ^ Dötz, K. H. (1983). "Carbon-carbon bond formation via carbonyl-carbene complexes". Pure Appl. Chem. 55: 1689. doi:10.1351/pac198355111689.
- ^ Dötz, K. H. (1990). New J. Chem. 14: 433–445.
- ^ http://www2.chemistry.msu.edu/faculty/wulff/myweb26/index.htm
- ^ Wulff, W. D.; Tang, P. C.; McCallum, J. S. (1981). "Regiochemistry of the reaction of chromium-carbene complexes with acetylenes". J. Am. Chem. Soc. 103: 7677–7678. doi:10.1021/ja00415a058.
- ^ Chamberlin, S.; Wulff, W. D. (1994). "Synthons for the Parent Vinyl Carbene Complex in the Benzannulation Reaction". J. Org. Chem. 59: 3047–3054. doi:10.1021/jo00090a024.
- ^ Rawat, M.; Wulff, W. D. (2004). "Total Synthesis of Carbazoquinocin C: Application of the o-Benzannulation of Fischer Carbene Complexes to Carbazole-3,4-quinone Alkaloids". Org. Lett. 6 (3): 329–332. doi:10.1021/ol0360445. PMID 14748585.
- ^ White, J. D.; Smits, H. (2005). "Application of the Dötz Reaction to Construction of a Major Portion of the Ansa Macrocycle (−)-Kendomycin". Org. Lett. 7 (2): 235–238. doi:10.1021/ol047779s. PMID 15646966.
Categories:- Addition reactions
- Carbon-carbon bond forming reactions
- Name reactions
Wikimedia Foundation. 2010.