- Sodium decavanadate
-
Sodium decavanadate 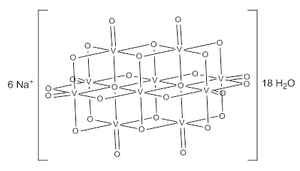
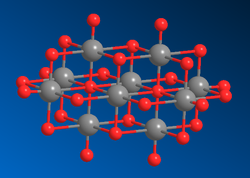
Identifiers CAS number 12315-57-0 
Properties Molecular formula Na6[V10O28] Molar mass 1419.6 g Appearance orange solid 
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium decavanadate (Na6[V10O28]) is an inorganic compound. It is the sodium salt of the decavanadate anion, [V10O28]6-. Decavanadate is unique among vanadates in that it forms an orange aqueous solution.[1] Numerous decavanadate salts and derivatives have been isolated and studied since 1956 when it was first characterized.[2]
Contents
Preparation
The preparation of decavanadate is achieved by acidifying an aqueous solution of vanadate.[1]
- 10Na3[VO4]•nH2O + 24HOAc → Na6[V10O28]•18H2O + 24NaOAc
The formation of decavanadate is optimized by maintaining a pH range of 4 - 7. The product can be purified with recrystallization in water. This synthesis avoids the introduction of foreign cations, except H+. Otherwise, a variety of mixed-cation decavanadates will form. Typical side products include metavanadate, [VO3]-, and hexavanadate, [V6O16]2-, ions.[1]
Structure
The decavanadate ion consists of six fused VO6 octahedra and has D2h symmetry.[2][3][4] The structure of Na6[V10O28]•18H2O has been confirmed with X-ray crystallography.[5]
 Figure 1: structure of decavanadate ion with equivalent V and O atoms indicated
Figure 1: structure of decavanadate ion with equivalent V and O atoms indicatedThe decavanadate anions contains three sets of equivalent V atoms (see fig. 1).[2] These include two central VO6 octahedra (Vc) and four each peripheral tetragonal-pyramidal VO5 groups (Va and Vb). There are seven unique groups of oxygen atoms (labeled A through G). Two of these (A) bridge to six V centers, four (B) bridge three V centers, fourteen of these (C, D & E) span edges between pairs of V centers, and eight (F & G) are peripheral.
The oxidation state of vanadium in decavanadate is +5. Vanadium can act as an oxidizing agent and form V (IV) or V (III).[6] Exposure to air maintains the vanadate, making them stable compounds.[6]
Acid-Base Properties
Aqueous vanadate (V) compounds undergo various self-condensation reactions.[6] Depending on pH, major vanadate anions in solution include VO22+, VO43-, V2O73-, V3O93-, V4O124-, and V10O266-. Many of these vanadate ions can be reversibly protonated.4 Decavanadate forms according to this equilibrium:[6][7]
- 10[VO2(OH2)4]+(aq) ⇌ H2V10O284-(aq) + 14H+(aq) + 32H2O
The pKa of this equilibrium depends on the ionic strength. For example, the pKa’s in 1 or 3 M sodium perchlorate are 6.8 and 5.5, respectively. Multiple protonations of the decavanadate ion can occur.
- H3V10O283-(aq) ⇌ H2V10O284-(aq) + H+(aq)
- H2V10O284-(aq) ⇌ HV10O285-(aq) + H+(aq)
- HV10O285-(aq) ⇌ V10O286-(aq) + H+(aq)
The symmetry of the various protonation states of the decavanadate ion has been examinated by 51V NMR spectroscopy.[4][6] Each species gives three signals; with slightly varying chemical shifts around -425, -506, and -523 ppm relative to VOCl3; suggesting that rapid proton exchange occurs resulting in equally symmetric species.[8] The three protonations of decavanadate have been shown to occur at the bridging oxygen centers, indicated as B and C in figure 1.[8]
Decavanadate is most stable in pH 4-7 region.[1][3][6] Solutions of vanadate turn bright orange at pH 6.5, indicating the presence of decavanadate. All other vanadates are colorless. Below pH 2.0 a brown V2O5 hydrate precipitates.[2][6]
- V10O286-(aq) + 6H+(aq) + 12H2 ⇌ 5V2O5•3H2Os
Related Decavanadates
Many decavanadate salts have been characterized. NH4+, Ca2+, Ba2+, Sr2+, and group I decavanadate salts are prepared by the acid-base reaction between V2O5 and the oxide, hydroxide, carbonate, or hydrogen carbonate of the desired positive ion.[1]
- 6NH3 + 5V2O5 + 3H2O ⇌ (NH4)6[V10O28]
Other Decavanadates:
- (NH4)6[V10O28]•6H2O[7]
- K6[V10O28]•9H2O[7]
- K6[V10O28]•10H2O[1][2][7]
- Ca3[V10O28]•16H2O[2][7]
- K2Mg2[V10O28]•16H2O[2][7]
- K2Zn2[V10O28]•16H2O[1][2][7]
- Cs2Mg2[V10O28]•16H2O[2]
- Cs4Na2[V10O28]•10H2O[9]
- K4Na2[V10O28]•16H2O[10]
- Sr3[V10O28]•22H2O[9]
- Ba3[V10O28]•19H2O[9]
- [(C6H5)4P]H3V10O28•4CH3CN[8]
References
- ^ a b c d e f g Johnson, G.; Murmann, R. K.; Deavin, R.; Griffith, W. P. (2007). "Sodium and Ammonium Decavanadates". Inorganic Synthesese 19: 140–145. doi:10.1002/9780470132500.ch32.
- ^ a b c d e f g h i Rossotti, F. J.; Rossotti, H. (1956). "Equilibrium Studies of Polyanions". Acta Chemica Scandinavica 10: 957–984. doi:10.3891/acta.chem.scand.10-0957.
- ^ a b Kustin, K.; Pessoa, J. C.; Crans, D. C. (2007). Vandadium: The Versatile Metal. Washington, D. C.: American Chemical Society. ISBN 978-0-8412-7446-4.
- ^ a b Rehder, D. (2008). Bioinorganic Vanadium Chemistry. Wiley & Sons. pp. 13–51. ISBN 978-0-470-06509-9.
- ^ Durif, P.A.; Averbuch-pouchot, M.T. (1980). "Structure d’un Décavanadate d’Hexasodium Hydraté". Acta Cryst. B36: 680–682. doi:10.1107/S0567740880004116.
- ^ a b c d e f g Tracey, A.S.; Crans, D.C. (1998). Vanadium Compounds. Washington D.C.: American Chemical Society. ISBN 0-8412-3589-9.
- ^ a b c d e f g Rossotti, F.J.C; Rossotti, H. (1956). "Equilibrium Studies of Polyanions". Acta Chemica Scandinavica. 10: 957–984. doi:10.3891/acta.chem.scand.10-0957.
- ^ a b c Day, V. W.; Klemperer, W. G.; Maltbie, D. J. (1987). "Where Are the Protons in H3V10O283-?". Am. Chem. Soc. 109: 2991–3002. doi:10.1021/ja00244a022.
- ^ a b c Dametto, A.C.; de Arauju, A.S.; de Souza Correa, R.; Guilherme, L.R.; Massabni, A.C. (2010). "Synthesis, infrared spectroscopy and crystal structure determination of a new decavanadate". J Chem Crystallogr. 40: 897–901. doi:10.1007/s10870-010-9759-x.
- ^ Matias, P.M.; Pessoa, J.C.; Duarte, M.T.; Maderia, C. (2000). "Tetrapotassium disodium decavanadate(V) decahydrate". Acta Cryst. 57: e75-e76. doi:10.1107/S0108270100001530.
Categories:- Polyoxometallates
- Vanadium compounds
Wikimedia Foundation. 2010.
