- Conformational proofreading
-
Conformational proofreading is a general mechanism of molecular recognition systems, in which introducing a structural mismatch between a molecular recognizer and its target enhances the recognition specificity and quality. [1] [2] [3] [4] [5]
Contents
Balancing correct and incorrect binding
Molecular recognition takes place in a noisy, crowded biological environment and the recognizer often has to cope with the task of selecting its target among a variety of similar competitors. For example, the ribosome has to select the correct tRNA that matches the mRNA codon among many structurally similar tRNAs. If the recognizer and its correct target match perfectly like a lock and a key, then the binding probability will be high since no deformation is required upon binding. At the same time, the recognizer might also bind to a competitor with a similar structure with high probability. Introducing a structural mismatch between the recognizer and reduces the binding probability to the correct target but reduces even more the binding probability to a similar wrong target and thus improves the specificity. Yet, introducing too much deformation drastically reduce binding probability to the correct target. Therefore, the optimal balance between maximizing the correct binding probability and minimizing the incorrect binding probability is achieved when the recognizer is slightly off target. This suggests that conformational changes during molecular recognition processes, such as the induced fit [6] mechanism, are advantageous for enhancing the specificity of recognition .
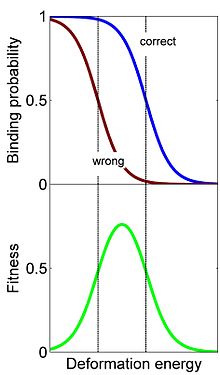 Conformational proofreading in homologous recombination. Top: The binding probability to homologous (correct) and non-homologous (wrong) DNA sequences decrease with the deformation energy barrier. The wrong binding probability decreases before the correct one. Bottom: As a result, the Fitness which is the difference, Fitness = Prob(Correct) - Prob(Wrong), is maximal at a non-zero deformation energy. This barrier is optimal in the sense that it significantly reduces the binding probability while keeping the correct binding probability roughly the same. Biochemical measurements of RecA-induced recombination suggest that the observed deformation is nearly optimal [3][4].
Conformational proofreading in homologous recombination. Top: The binding probability to homologous (correct) and non-homologous (wrong) DNA sequences decrease with the deformation energy barrier. The wrong binding probability decreases before the correct one. Bottom: As a result, the Fitness which is the difference, Fitness = Prob(Correct) - Prob(Wrong), is maximal at a non-zero deformation energy. This barrier is optimal in the sense that it significantly reduces the binding probability while keeping the correct binding probability roughly the same. Biochemical measurements of RecA-induced recombination suggest that the observed deformation is nearly optimal [3][4].
Conformational Proofreading in Homologous Recombination
The mechanism of conformational proofreading is utilized in the system of homologous recombination to discern between similar DNA sequences [3][4]. Homologous recombination facilitates the exchange of genetic material between homologous DNA molecules. This crucial process requires detecting a specific homologous DNA sequence within a huge variety of heterologous sequences. The detection is mediated by RecA in E. coli, or members of its superfamily in other organisms. RecA first polymerizes along a stretch of single-stranded DNA, and then this protein-DNA filament searches for homology along double-stranded DNA. In the RecA-DNA filament, the distance between bases increases significantly with respect to the bare 3.4 Å in the double-strand (by 50% on average [7]). This sets a significant energetic barrier on the search, since the double-stranded DNA has to stretch by the same magnitude to check for homology. By formulating the DNA recognition process as a signal detection problem, it was shown that the experimentally observed RecA-induced DNA deformation and the binding energetics are fine-tuned to ensure optimal sequence detection. The amount of deformation is such that binding to homologous DNA sequences only slightly decreases, while binding to wrong sequences decreases significantly.
Relation to kinetic proofreading
In the kinetic proofreading [8] [9] schema, a time delay (equivalently, an irreversible intermediate stage) is introduced during the formation of the correct or incorrect complexes. This time delay reduces the production rates of both complexes but enhances the fidelity beyond the equilibrium limit. The irreversibility of the scheme requires an energy source. The time delay in kinetic proofreading is analogous to the spatial difference in conformational proofreading. However, the conformational proofreading is an equilibrium scheme that does not consume energy.
References
- ^ Savir Y & Tlusty T (2007). Scalas, Enrico. ed. "Conformational Proofreading: The Impact of Conformational Changes on the Specificity of Molecular Recognition". PLoS ONE 2 (5): e468. doi:10.1371/journal.pone.0000468. PMC 1868595. PMID 17520027. http://www.weizmann.ac.il/complex/tlusty/papers/PLoSONE2007.pdf.
- ^ Savir Y & Tlusty T (2008). "Optimal Design of a Molecular Recognizer : Molecular Recognition as a Bayesian Signal Detection Problem". IEEE J Sel Topics Signal Process 2 (3): 390–399. doi:10.1109/JSTSP.2008.923859. http://www.weizmann.ac.il/complex/tlusty/papers/IEEE2008.pdf.
- ^ a b c Savir Y & Tlusty T (2010). "RecA-mediated homology search as a nearly optimal signal detection system". Molecular Cell 40 (3): 388–96. doi:10.1016/j.molcel.2010.10.020. PMID 21070965. http://www.weizmann.ac.il/complex/tlusty/papers/MolCell2010.pdf.
- ^ a b c Rambo RP, Williams GJ, Tainer JA. (2010). "Achieving Fidelity in Homologous Recombination Despite Extreme Complexity: Informed Decisions by Molecular Profiling". Molecular Cell 40 (3): 347–48. doi:10.1016/j.molcel.2010.10.032. PMC 3003302. PMID 21070960. http://www.weizmann.ac.il/complex/tlusty/papers/MolCell2010B.pdf.
- ^ Alon U (2008). "Journal Club". Nature 453 (7196): 701. doi:10.1038/453701e. http://www.weizmann.ac.il/complex/tlusty/PR/JCNature2008.pdf.
- ^ Koshland, D. E. (1958). "Application of a Theory of Enzyme Specificity to Protein Synthesis". Proc Natl Acad Sci U S A 44 (2): 98–104. doi:10.1073/pnas.44.2.98. PMC 335371. PMID 16590179. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=335371.
- ^ Chen Z, Yang H, Pavletich NP. (2008). "Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures". Nature 453 (7194): 489–4. doi:10.1038/nature06971. PMID 18497818.
- ^ Hopfield JJ (1974). "Kinetic Proofreading: A New Mechanism for Reducing Errors in Biosynthetic Processes Requiring High Specificity". Proc Natl Acad Sci U S A 71 (10): 4135–4139. doi:10.1073/pnas.71.10.4135. PMC 434344. PMID 4530290. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=434344.
- ^ Ninio J (1975). "Kinetic amplification of enzyme discrimination Biochimie". Biochimie 57 (5): 587–595. doi:10.1016/S0300-9084(75)80139-8. PMID 1182215. http://www.sciencedirect.com/science/article/pii/S0300908475801398.
External links
Categories:- Mathematical and theoretical biology
- Biomolecules
- Enzymes
Wikimedia Foundation. 2010.
