- Oxysterol-binding protein
-
Oxysterol-binding protein 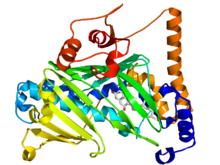
Crystallographic structure of the oxysterol-binding protein (rainbow color cartoon, N-terminus = blue, C-terminus = red) bound to 7-hydroxycholesterol (stick diagram, carbon = white, oxygen = red).[1] Identifiers Symbol Oxysterol_BP Pfam PF01237 InterPro IPR000648 PROSITE PDOC00774 OPM protein 1zi7 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Oxysterol-binding proteins are evolutionary related proteins involved with sterol synthesis and/or its regulation [2]. These include mammalian oxysterol-binding protein (OSBP), a protein of about 800 amino-acid residues that binds a variety of oxysterols (oxygenated derivatives of cholesterol); yeast OSH1, a protein of 859 residues that also plays a role in ergosterol synthesis; and yeast proteins HES1 and KES1, highly related proteins of 434 residues that seem to play a role in ergosterol synthesis[3]
References
- ^ PDB 1ZHT; Im YJ, Raychaudhuri S, Prinz WA, Hurley JH (September 2005). "Structural mechanism for sterol sensing and transport by OSBP-related proteins". Nature 437 (7055): 154–8. doi:10.1038/nature03923. PMC 1431608. PMID 16136145. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1431608.
- ^ Bussey H, Fortin N, Jiang B, Brown JL, Sheraton J (1994). "A new family of yeast genes implicated in ergosterol synthesis is related to the human oxysterol binding protein". Yeast 10 (3): 341–353. doi:10.1002/yea.320100307. PMID 8017104.
- ^ Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA; J Cell Biol 2002;157:63-77. PubMed
Human proteins containing this domain
OBPH1; OSBP; OSBP2; OSBPL10; OSBPL11; OSBPL1A; OSBPL2; OSBPL3; OSBPL5; OSBPL6; OSBPL7; OSBPL8; OSBPL9;
This article includes text from the public domain Pfam and InterPro IPR000648

This membrane protein-related article is a stub. You can help Wikipedia by expanding it.
