- Sodium
-
This article is about the chemical element. For the PlayStation Home game, see Sodium (PlayStation Home).
neon ← sodium → magnesium Li
↑
Na
↓
KAppearance silvery white metallic


Spectral lines of sodiumGeneral properties Name, symbol, number sodium, Na, 11 Pronunciation /ˈsoʊdiəm/ soh-dee-əm Element category alkali metal Group, period, block 1, 3, s Standard atomic weight 22.98976928(2) Electron configuration [Ne] 3s1 Electrons per shell 2,8,1 (Image) Physical properties Phase solid Density (near r.t.) 0.968 g·cm−3 Liquid density at m.p. 0.927 g·cm−3 Melting point 370.87 K, 97.72 °C, 207.9 °F Boiling point 1156 K, 883 °C, 1621 °F Critical point (extrapolated)
2573 K, 35 MPaHeat of fusion 2.60 kJ·mol−1 Heat of vaporization 97.42 kJ·mol−1 Molar heat capacity 28.230 J·mol−1·K−1 Vapor pressure P (Pa) 1 10 100 1 k 10 k 100 k at T (K) 554 617 697 802 946 1153 Atomic properties Oxidation states +1, 0, -1
(strongly basic oxide)Electronegativity 0.93 (Pauling scale) Ionization energies
(more)1st: 495.8 kJ·mol−1 2nd: 4562 kJ·mol−1 3rd: 6910.3 kJ·mol−1 Atomic radius 186 pm Covalent radius 166±9 pm Van der Waals radius 227 pm Miscellanea Crystal structure body-centered cubic Magnetic ordering paramagnetic Electrical resistivity (20 °C) 47.7 nΩ·m Thermal conductivity 142 W·m−1·K−1 Thermal expansion (25 °C) 71 µm·m−1·K−1 Speed of sound (thin rod) (20 °C) 3200 m·s−1 Young's modulus 10 GPa Shear modulus 3.3 GPa Bulk modulus 6.3 GPa Mohs hardness 0.5 Brinell hardness 0.69 MPa CAS registry number 7440-23-5 Most stable isotopes Main article: Isotopes of sodium iso NA half-life DM DE (MeV) DP 22Na trace 2.602 y β+→γ 0.5454 22Ne* 1.27453(2)[1] 22Ne ε→γ - 22Ne* 1.27453(2) 22Ne β+ 1.8200 22Ne 23Na 100% 23Na is stable with 12 neutrons Sodium (
 /ˈsoʊdiəm/ soh-dee-əm) is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride. Many salts of sodium are highly soluble in water and are thus present in significant quantities in the Earth's bodies of water.
/ˈsoʊdiəm/ soh-dee-əm) is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride. Many salts of sodium are highly soluble in water and are thus present in significant quantities in the Earth's bodies of water.Many sodium compounds are useful, such as sodium hydroxide (lye) for soapmaking, and sodium chloride for use as a deicing agent and a nutrient. Sodium is an essential element for all animals and some plants. In animals, sodium ions are used against potassium ions to build up charges on cell membranes, allowing transmission of nerve impulses when the charge is dissipated; hence, it is thus classified as a dietary inorganic macro-mineral.
The free metal, elemental sodium, does not occur in nature but must be prepared from sodium compounds. Elemental sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. The same ion is also a component of many minerals, such as sodium nitrate.
Contents
Characteristics
Physical
 A positive flame test for sodium has a bright yellow color.
A positive flame test for sodium has a bright yellow color.
Sodium can be cut with a knife at room temperature; freshly exposed sodium has a bright, silvery luster that rapidly tarnishes and forms a white oxide layer if left exposed to air. Although the density of alkali metals generally increases along with atomic number, sodium is denser than potassium and is a fairly good heat conductor. At 1.5 Mbar, sodium becomes black, changing to red transparent at 1.9 Mbar, and finally clear transparent at 3 Mbar; these high pressure allotropes are insulators and have the electron as an anion.[2]
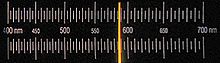
When sodium or its compounds are introduced into a flame, they turn it bright yellow. This is because the heat excites sodium atoms and moves their valence electrons from the 3s orbital to the 3p orbital; as those electrons fall back to 3s, they emit a photon with a wavelength corresponding to the D line at 589.3 nm. Spin-orbit interactions of the valence electron in the 3p orbital cause the D line to split into the D1 (589.6 nm) and D2 (589.0 nm) lines; hyperfine structures of both orbitals lead to many more lines.[3][4][5] A practical use for lasers emitting light at the D line is to create artificial laser guide stars that assist in the adaptive optics for large land-based visible light telescopes.
Chemical
As predicted by the periodic table, sodium is generally less reactive than potassium and more reactive than lithium.[6] Like all the alkali metals, it reacts exothermically with water, to the point that sufficiently large pieces melt to a sphere and then explode; this reaction produces caustic sodium hydroxide and flammable hydrogen gas. When burned in air, depending on oxygen concentration, it forms either Na2O2 or Na2O; if burned in pure, pressurised oxygen, NaO2 results.
Isotopes
Main article: Isotopes of sodium20 isotopes of sodium are known, but only 23Na is stable. Two radioactive, cosmogenic isotopes are the byproduct of cosmic ray spallation: 22Na with a half-life of 2.6 years and 24Na with a half-life of 15 hours; all other isotopes have a half-life of less than one minute.[7] Two nuclear isomers have been discovered, the longer-lived one being 24mNa with a half-life of around 20.2 microseconds. Acute neutron radiation, such as from a nuclear criticality accident, converts some of the stable 23Na in human blood to 24Na; by measuring the concentration of 24Na in relation to 23Na, the neutron radiation dosage of the victim can be calculated.[8]
Occurence
23Na is created through the carbon-burning process by fusing two carbon atoms together; this requires temperatures above 600 megakelvins and a star with at least three solar masses.[9] The Earth's crust has 2.6% sodium by weight, making it the sixth most abundant element there.[10] It is found in many different, mostly soluble such as salt, amphibole, and zeolite; owing to its high reactivity, it is never found by itself. The insolubility of certain minerals such as cryolite and feldspar arises from their polymeric anions, which in the case of feldspar is a polysilicate. In the interstellar medium, sodium is identified by the D line; though it has a high vaporization temperature, its abundance allowed it to be detected by Mariner 10 in Mercury's atmosphere.[11]
Compounds
See also Category: Sodium compounds.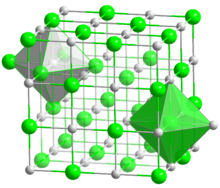 Structure of sodium chloride, showing octahedral coordination around Na+ and Cl- centres. This framework disintegrates upon dissolution in water and reassembles upon evaporation.
Structure of sodium chloride, showing octahedral coordination around Na+ and Cl- centres. This framework disintegrates upon dissolution in water and reassembles upon evaporation.
Sodium compounds are of immense commercial importance, being particularly central to industries producing glass, paper, soap, and textiles.[12] The sodium compounds that are the most important are common salt (NaCl), soda ash (Na2CO3), baking soda (NaHCO3), caustic soda (NaOH), sodium nitrate (NaNO3), di- and tri-sodium phosphates, sodium thiosulfate (Na2S2O3·5H2O), and borax (Na2B4O7·10H2O).[13] In its compounds, sodium is usually connected via ionic bonding to water and the anions. In this way, it is viewed as a weak but "hard" Lewis acid.
Aqueous solutions
Sodium tends to form water-soluble compounds, such as halides, sulfates, nitrates, carboxylates and carbonates. The main species in water are the aquo complexes [Na(H2O)n]+ where n = 4-6.[14]
The high affinity of sodium for oxygen-based ligands is the basis of crown ethers. Functionally related but more complex ligands are several macrolide antibiotics, which function by interfering with the transport of Na+ in the infecting organism.
Examples of sodium salts precipitating from water solution are rare, and most feature large or organic anions. illustrative sodium salts exhibiting low solubility include sodium bismuthate (NaBiO3).[15] Since sodium salts are so soluble in water, they are usually isolated as solids by evaporation of these aqueous solutions or by precipitation with an organic solvent. For example, 360 g of sodium chloride will dissolve in water. The addition of ethanol to such solutions causes solid NaCl to separate (precipitate), because only 0.35 g of sodium chloride will dissolve in the alcohol.[16]
Organosodium compounds
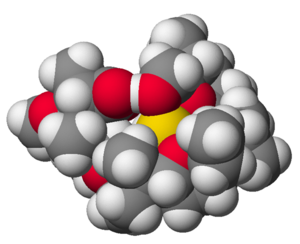 The structure of the complex of sodium (Na+) and the antibiotic monensin-A.
The structure of the complex of sodium (Na+) and the antibiotic monensin-A.
Many organosodium compounds have been prepared. Because of the high polarity of the C-Na bonds, they are treated sources of carbanions. Some well known derivatives include sodium cyclopentadienide (NaC5H5) and trityl sodium ((C6H5)3CNa).[17]
History
Salt has been an important commodity in human activities, as testified by the English word salary, referring to salarium, the wafers of salt sometimes given to Roman soldiers along with their other wages. In medieval Europe a compound of sodium with the Latin name of sodanum was used as a headache remedy. The name sodium probably originates from the Arabic word suda meaning headache as the headache-alleviating properties of sodium carbonate or soda were well known in early times.[18]
Sodium's chemical abbreviation Na was first published by Jöns Jakob Berzelius in his system of atomic symbols (Thomas Thomson, Annals of Philosophy[19]) and is a contraction of the element's new Latin name natrium which refers to the Egyptian natron,[18] the word for a natural mineral salt whose primary ingredient is hydrated sodium carbonate. Hydrated sodium carbonate historically had several important industrial and household uses later eclipsed by soda ash, baking soda and other sodium compounds.
Although sodium (sometimes called "soda" in English) has long been recognized in compounds, the metal itself was not isolated until 1807 by Humphry Davy through the electrolysis of caustic soda.[20][21] Sodium imparts an intense yellow color to flames; as early as 1860, Kirchhoff and Bunsen noted the high sensitivity of a sodium flame test, and stated in Annalen der Physik und Chemie:[22]
“ In a corner of our 60 m3 room farthest away from the apparatus, we exploded 3 mg. of sodium chlorate with milk sugar while observing the nonluminous flame before the slit. After a while, it glowed a bright yellow and showed a strong sodium line that disappeared only after 10 minutes. From the weight of the sodium salt and the volume of air in the room, we easily calculate that one part by weight of air could not contain more than 1/20 millionth weight of sodium. ” Commercial production
Enjoying rather specialized applications, only about 100,000 tonnes of metallic sodium are produced annually.[12] Metallic sodium was first produced commercially in 1855 by carbothermal reduction of sodium carbonate at 1100 °C, in what is known as the Deville process:[23][24][25]
- Na2CO3 + 2 C → 2 Na + 3 CO
A related process based on the reduction of sodium hydroxide was developed in 1886.[23]
Sodium is now produced commercially through the electrolysis of molten sodium chloride, based on a process patented in 1924.[26][27] This is done in a Downs Cell in which the NaCl is mixed with calcium chloride to lower the melting point below 700 °C. As calcium is less electropositive than sodium, no calcium will be formed at the anode. This method is less expensive than the previous Castner process of electrolyzing sodium hydroxide.
Sodium metal in reagent-grade sold for about US$1.50/pound ($3.30/kg) in 2009 when purchased in tonne quantities. Lower purity metal sells for considerably less. The market in this metal is volatile due to the difficulty in its storage and shipping. It must be stored under a dry inert gas atmosphere or anhydrous mineral oil to prevent the formation of a surface layer of sodium oxide or sodium superoxide. These oxides can react violently in the presence of organic materials. Sodium will also burn violently when heated in air.[28]
Smaller quantities of sodium, such as a kilogram, cost far more, in the range of US$165/kg. The high cost is partially due to the expense of shipping hazardous material.[29]
Applications
The dominant applications of sodium are for its many chemical compounds. In terms of scale, the chloride, hydroxide, and carbonate are produced on megatons/y. Although not produced on comparable tonnage, many important medicine formulations are sodium or potassium is a common modification to improve bioavailability.[12]
Soap
Most soaps are sodium salts of fatty acids. Sodium soaps are harder (higher melting) soaps than potassium soaps.[13] Sodium chloride is extensively used as a de-icing agent and as a preservative; sodium bicarbonate is mainly used for cooking.
Metallic sodium
Sodium in its metallic form is mainly consumed for the production of the following chemical compounds: sodium hydride (NaH, precursor to sodium borohydride), sodium amide (NaNH2 (precursor to sodium azide and indigo), triphenylphosphine (P(C6H5)3, reagent for the production of vitamin A). In former times, the production of tetraethyllead and titanium metal were the major consumers of metallic sodium, but these technologies have been discontinued. Consequently the production of sodium declined since the 1970's.[12] Some continuing applications include:
- In certain alloys to improve their structure.
- To descale metal (make its surface smooth).[30][31]
- To purify molten metals. Especially where carbon, aluminium etc. cannot be used for reducing metallic compounds.
- Sodium vapor lamps are an efficient means of producing light from electricity and they are often used for street lighting in cities. Low-pressure sodium lamps give a distinctive yellow-orange light which consists primarily of the twin sodium D lines. High-pressure sodium lamps give a more natural peach-colored light, composed of wavelengths spread much more widely across the spectrum.[32]
- In organic synthesis, sodium is used as a reducing agent, for example in the Birch reduction. It is also used for preparing Na-Extract which is a major method of identification of elements present in organic compound.
- In chemistry, sodium is often used either alone or with potassium in an alloy, NaK as a desiccant for drying solvents. Used with benzophenone, it forms an intense blue coloration when the solvent is dry and oxygen-free.
- The sodium fusion test uses sodium's high reactivity, low melting point, and the near-universal solubility of its compounds, to qualitatively analyze compounds.
Heat exchanger
As a heat transfer fluid, sodium is found in sodium-cooled fast reactors[33] and inside the hollow valves of high-performance internal combustion engines.
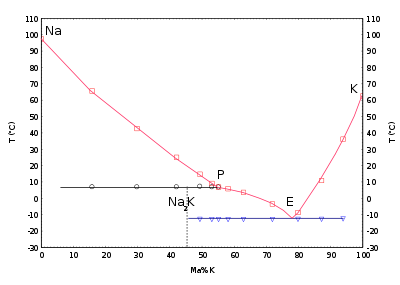 NaK phase diagram, showing the melting point of sodium as a function of percentage of potassium in it.
NaK phase diagram, showing the melting point of sodium as a function of percentage of potassium in it.
Molten sodium is used as a coolant in some types of fast neutron reactors. It has a low neutron absorption cross section, which is required to achieve a high enough neutron flux, and has excellent thermal conductivity. Its high boiling point allows the reactor to operate at ambient pressure. However, using sodium poses certain challenges. The molten metal will readily burn in air and react violently with water, liberating explosive hydrogen. During reactor operation, a small amount of sodium-24 is formed as a result of neutron activation, making the coolant radioactive.
Sodium leaks and fires were a significant operational problem in the first large sodium-cooled fast reactors, causing extended shutdowns at the Monju Nuclear Power Plant and Beloyarsk Nuclear Power Plant.
Where reactors need to be frequently shut down, as is the case with some research reactors, the alloy of sodium and potassium called NaK is used. It melts at −11 °C, so cooling pipes will not freeze at room temperature. Extra precautions against coolant leaks need to be taken in case of NaK, because molten potassium will spontaneously ignite when exposed to air.
The phase diagram with potassium shows that the mixtures with potassium are liquid at room temperature in a wide concentration range. A compound Na2K melts at 7 °C. The eutectic mixture with a potassium content of 77 % gives a melting point at −12.6 °C.[34]
Botany
Although sodium is not considered as important as potassium, it is considered as a micronutrient in most plants as it is necessary in the metabolism of some C4 plants, such as Rhodes grass, amaranth, Joseph's coat, and pearl millet.[35] Within these C4 plants, sodium is used in the regeneration of phosphoenolpyruvate (PEP) and the synthesis of chlorophyll. In addition, the presence of sodium can offset potassium requirements in many plants by substituting in several roles, such as: maintaining turgor pressure, serving as an accompanying cation in long distance transport, and aiding in stomatal opening and closing.[36]
Increasing soil salinity, osmotic stress and sodium toxicity in plants, especially in agricultural crops, have become worldwide phenomena. High levels of sodium in the soil solution limit the plants' ability to uptake water due to decreased soil water potential and, therefore, may result in wilting of the plant. In addition, excess sodium within the cytoplasm of plant cells can lead to enzyme inhibition, which may result in symptoms such as necrosis, chlorosis, and possible plant death.[37] To avoid such symptoms, plants have developed methods to combat high sodium levels, such as: mechanisms limiting sodium uptake by roots, compartmentalization of sodium in cell vacuoles, and control of sodium in long distance transport.[38] Many plants store excess sodium in old plant tissue, limiting damage to new growth.
Biological role
Sodium is an essential nutrient that regulates blood volume and blood pressure, "maintains the right balance of fluids in the body, transmits nerve impulses, and influences the contraction and relaxation of muscles".[39] It is also necessary for maintaining osmotic equilibrium and the acid-base balance. The minimum physiological requirement for sodium is only 500 milligrams per day.[40] However, according to the American Heart Association, in order to "ensure nutrient adequacy and replace sweat losses a healthy adult needs 1,500 milligrams of sodium per day" or 2/3 of a teaspoon.[41]
Maintaining body fluid volume in animals
Main articles: Renin-angiotensin system and atrial natriuretic peptideThe serum sodium and urine sodium play important roles in medicine, both in the maintenance of sodium and total body fluid homeostasis, and in the diagnosis of disorders causing homeostatic disruption of salt/sodium and water balance.
In mammals, decreases in blood pressure and decreases in sodium concentration sensed within the kidney result in the production of renin, a hormone which acts in a number of ways, one of them being to act indirectly to cause the generation of aldosterone, a hormone which decreases the excretion of sodium in the urine. As the body of the mammal retains more sodium, other osmoregulation systems which sense osmotic pressure in part from the concentration of sodium and water in the blood, act to generate antidiuretic hormone. This, in turn, causes the body to retain water, thus helping to restore the body's total amount of fluid.
There is also a counterbalancing system, which senses volume. As fluid is retained, receptors in the heart and vessels which sense distension and pressure, cause production of atrial natriuretic peptide, which is named in part for the Latin word for sodium. This hormone acts in various ways to cause the body to lose sodium in the urine. This causes the body's osmotic balance to drop (as low concentration of sodium is sensed directly), which in turn causes the osmoregulation system to excrete the "excess" water. The net effect is to return the body's total fluid levels back toward normal.
Maintaining electric potential in animal tissues
Main article: Action potentialSodium cations are important in neuron (brain and nerve) function, and in influencing osmotic balance between cells and the interstitial fluid, with their distribution mediated in all animals (but not in all plants) by the so-called Na+/K+-ATPase pump.[42] Sodium is the chief cation in fluid residing outside cells in the mammalian body (the so-called extracellular compartment), with relatively little sodium residing inside cells. The volume of extracellular fluid is typically 15 liters in a 70 kg human, and the 50 grams of sodium it contains is about 90% of the body's total sodium content.
Dietary uses
The most common sodium salt, sodium chloride ('table salt' or 'common salt'), is used for seasoning and warm-climate food preservation, such as pickling and making jerky (the high osmotic content of salt inhibits bacterial and fungal growth). The human requirement for sodium in the diet is about 1.5 grams per day.[43] This is less than a tenth of the sodium in many diets "seasoned to taste." Most people consume far more sodium than is physiologically needed. Low sodium intake may lead to sodium deficiency (hyponatremia).
Persons suffering from severe dehydration caused by diarrhea, such as that by cholera, can be treated with oral rehydration therapy, in which they drink a solution of sodium chloride, potassium chloride and glucose. This simple, effective therapy saves the lives of millions of children annually in the developing world.[citation needed]
US consumption and guidelines
The 2010 dietary guidelines of the United States Department of Agriculture (USDA) recommend to decrease the sodium consumption to less than 2.3 g per day (1 teaspoon of salt). This target is lowered to 1.5 g/day (2/3 teaspoon) for "salt sensitive populations", which includes individuals older than 51, African Americans or those who have hypertension, diabetes, or chronic kidney diseases, and comprises about 50% of the US population.[44] The American Heart Association (AHA) proposes to lower the upper threshold to 2 g/day by 2013 and to 1.5 g/day by 2020 for everyone.[41]
According to the USDA, consumption of sodium in the US is disproportionately greater than the recommended intake, and AHA estimates the daily intake of sodium as 3.4 g/day. NHANES reported that in 2005–2006 the lowest consumption was by 2–5 years old females (~2.1 g/day) and the highest was for 30–39 years old males (~4.7 g/day); males consumed more than 2.3 g/day regardless of age and more than females of the same age group.[44]
Dietary sodium exposure
Dietary sodium comes from table salt, natural sources and processed foods. Processed foods contain an elevated amount of sodium, along with fast food and also foods that do not taste salty such as cheeses. Primary exposure to sodium in North America (75%) comes from highly processed foods or frozen meals.[45] Secondary exposure is correlated with eating at restaurants, predominately fast food chains. Tertiary exposure is experienced when individuals add table salt to their meals during preparation or after they are cooked. Thus simply removing salt shakers from dining tables will not resolve the problem of excessive sodium intake, and the cooperation of food manufacturers and restaurants to reduce the sodium content will be essential. The AHA urges food manufacturers and restaurants to reduce the salt they add to food by 50% over the next 10 years.[41]
Economic impact
Regular consumption of more than 2.3 g/day of sodium promotes such health problems as elevated blood pressure and cardiovascular disease. Excessive sodium consumption causes 9–17% of cases of hypertension.[46] Approximately 65 million adults in the United States, and 1 billion adults throughout the world have hypertension.[47] Worldwide, 7.6 million premature deaths (about 13.5% of the global total) and 92 million disability adjusted life years (6.0% of the global total) can be attributed to high blood pressure.[48] In 2010, the AHA estimated that the costs associated with hypertension and cardiovascular disease amounted to $503 billion dollars, that is people with cardiovascular disease and hypertension cost more than any other diagnostic group. AHA speculates that billions of dollars and approximately 8.5 million deaths between 2006 and 2015 could be averted globally if salt consumption was reduced in accordance with the 2010 dietary guidelines.[49]
Precautions
Care is required in handling elemental sodium, as it is potentially explosive and generates flammable hydrogen and caustic sodium hydroxide upon contact with water; powdered sodium may combust spontaneously in air or oxygen.[50] Excess sodium can be safely removed by hydrolysis in a ventilated cabinet; this is typically done by sequential treatment with isopropanol, ethanol and water. Isopropanol reacts very slowly, generating the corresponding alkoxide and hydrogen.[51] Fire extinguishers based on water accelerate sodium fires; those based on carbon dioxide and bromochlorodifluoromethane lose their effectiveness when they dissipate. An effective extinguishing agent is Met-L-X, which comprises approximately 5% Saran in sodium chloride together with flow agents; it is most commonly hand-applied with a scoop. Other materials include Lith+, which has graphite powder and an organophosphate flame retardant, and dry sand.
See also
References
- ^ Endt, P. M. (12/1990). "Energy levels of A = 21–44 nuclei (VII)". Nuclear Physics A 521: 1–400. Bibcode 1990NuPhA.521....1E. doi:10.1016/0375-9474(90)90598-G.
- ^ Gatti, M.; Tokatly, I.; Rubio, A. (2010). "Sodium: A Charge-Transfer Insulator at High Pressures". Physical Review Letters 104 (21): 216–404. Bibcode 2010PhRvL.104u6404G. doi:10.1103/PhysRevLett.104.216404.
- ^ Citron, M. L.; Gabel, C.; Stroud, C. (1977). "Experimental study of power broadening in a two level atom". Physical Review A 16 (4): 1507. Bibcode 1977PhRvA..16.1507C. doi:10.1103/PhysRevA.16.1507.
- ^ Steck, Daniel A.. "Sodium D. Line Data" (PDF). Los Alamos National Laboratory (technical report). http://george.ph.utexas.edu/~dsteck/alkalidata/sodiumnumbers.pdf.
- ^ Milonni, Peter W.; Eberly, Joseph H. (2009-06-12). "Example: Sodium Vapor". Laser Physics. pp. 118–120. ISBN 9780470387719. http://books.google.de/books?id=yIUjyETRt8cC&pg=PA118.
- ^ De Leon, N. "Reactivity of Alkali Metals". Indiana University Northwest. http://www.iun.edu/~cpanhd/C101webnotes/modern-atomic-theory/alkali-reac.html. Retrieved 2007-12-07.
- ^ Audi, Georges (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. Bibcode 2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Sanders, F. W.; Auxier, J. A. (1962). "Neutron Activation of Sodium in Anthropomorphous Phantoms". HealthPhysics 8 (4): 371–379. doi:10.1097/00004032-196208000-00005. PMID 14496815.
- ^ Denisenkov, P. A.; Ivanov, V. V. (1987). "Sodium Synthesis in Hydrogen Burning Stars". Soviet Astronomy Letters 13: 214. Bibcode 1987SvAL...13..214D.
- ^ Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Tjrhonsen, Dietrick E. (1985-08-17). "Sodium found in Mercury's atmosphere". BNET. http://findarticles.com/p/articles/mi_m1200/is_v128/ai_3898126. Retrieved 2008-09-18.
- ^ a b c d Alfred Klemm, Gabriele Hartmann, Ludwig Lange, "Sodium and Sodium Alloys" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a24 277
- ^ a b Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Natrium" (in German). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 931–943. ISBN 3-11-007511-3.
- ^ S. F. Lincoln, D. T. Richens, A. G. Sykes "Metal Aqua Ions" Comprehensive Coordination Chemistry II Volume 1, Pages 515-555. doi:10.1016/B0-08-043748-6/01055-0
- ^ Dean, John Aurie; Lange, Norbert Adolph (1998). Lange's Handbook of Chemistry. McGraw-Hill. ISBN 0070163847.
- ^ Burgess, J. (1978). Metal Ions in Solution. New York: Ellis Horwood. ISBN 0-85312-027-7.
- ^ W. B. Renfrow, Jr and C. R. Hauser (1943), "Triphenylmethylsodium", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0607; Coll. Vol. 2: 607
- ^ a b Newton, David E. (1999). Baker, Lawrence W.. ed. Chemical Elements. ISBN 978-0-7876-2847-5. OCLC 39778687.
- ^ van der Krogt, Peter. "Elementymology & Elements Multidict". http://elements.vanderkrogt.net/element.php?sym=Na. Retrieved 2007-06-08.
- ^ Davy, Humphry (1808). "On some new phenomena of chemical changes produced by electricity, particularly the decomposition of the fixed alkalies, and the exhibition of the new substances which constitute their bases; and on the general nature of alkaline bodies". Philosophical Transactions of the Royal Society of London 98: 1–44. doi:10.1098/rstl.1808.0001. http://books.google.com/?id=gpwEAAAAYAAJ&pg=PA57#v=onepage&q.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements. IX. Three alkali metals: Potassium, sodium, and lithium". Journal of Chemical Education 9 (6): 1035. Bibcode 1932JChEd...9.1035W. doi:10.1021/ed009p1035.
- ^ Kirchhoff, G.; Bunsen, R. (1860). "Chemische Analyse durch Spectralbeobachtungen". Annalen der Physik und Chemie 186 (6): 161–189. Bibcode 1860AnP...186..161K. doi:10.1002/andp.18601860602.
- ^ a b Eggeman, Tim (2007). Sodium and Sodium Alloys. John Wiley & Sons. doi:10.1002/0471238961.1915040912051311.a01.pub3.
- ^ Oesper, R. E.; Lemay, P. (1950). "Henri Sainte-Claire Deville,1818-1881". Chymia 3: 205–221. JSTOR 27757153.
- ^ Banks, Alton (1990). "Sodium". Journal of Chemical Education 67 (12): 1046. Bibcode 1990JChEd..67.1046B. doi:10.1021/ed067p1046.
- ^ Pauling, Linus, General Chemistry, 1970 ed., Dover Publications
- ^ "Los Alamos National Laboratory – Sodium". http://periodic.lanl.gov/11.shtml. Retrieved 2007-06-08.
- ^ "Sodium Metal 99.97% Purity". Galliumsource.com. http://www.galliumsource.com/index.html. Retrieved 2010-11-27.
- ^ "007-Sodium Metal". Mcssl.com. http://www.mcssl.com/store/gallium-source/sodium-metal. Retrieved 2010-11-27.
- ^ National Association of Drop Forgers and Stampers (1957). Metal treatment and drop forging. http://books.google.com/?id=kyVWAAAAYAAJ&dq=sodium+descale+metal&q=METALLIC+SODIUM+++DESCALING+SEVERAL#search_anchor.
- ^ Harris, Jay C. (1949). Metal cleaning bibliographical abstracts. p. 76. http://books.google.de/books?id=LI4KmKqca78C&pg=PA76.
- ^ Lindsey, Jack L. (1997). Applied illumination engineering. p. 112. ISBN 9780881732122. http://books.google.com/books?id=0d7u9Nr33zIC&pg=PA112.
- ^ Sodium as a Fast Reactor Coolant presented by Thomas H. Fanning. Nuclear Engineering Division. U.S. Department of Energy. U.S. Nuclear Regulatory Commission. Topical Seminar Series on Sodium Fast Reactors. May 3, 2007
- ^ van Rossen, G. L. C. M.; van Bleiswijk, H. (1912). "Über das Zustandsdiagramm der Kalium-Natriumlegierungen". Z. Anorg. Chem. 74: 152–156. doi:10.1002/zaac.19120740115.
- ^ Kering, M. K. "Manganese Nutrition and Photosynthesis in NAD-malic enzyme C4 plants". Ph.D. dissertation, University of Missouri-Columbia, 2008. Retrieved 2011-11-09.
- ^ Subbarao, G. V.; Ito, O.; Berry, W. L.; Wheeler, R. M. (2003). "Sodium—A Functional Plant Nutrient". Critical Reviews in Plant Sciences 22 (5): 391–416. doi:10.1080/07352680390243495.
- ^ Zhu, J. K. (2001). "Plant salt tolerance". Trends in plant science 6 (2): 66–71. doi:10.1016/S1360-1385(00)01838-0. PMID 11173290.
- ^ "Plants and salt ion toxicity". Plant Biology. Accessed 11/02/2010
- ^ Sodium: How to tame your salt habit now, Mayo Clinic
- ^ Sodium, Northewestern University
- ^ a b c 2010 Dietary Guidelines, page 4, The American Heart Association, January 23, 2009
- ^ Campbell, Neil (1987). Biology. Benjamin/Cummings. p. 795. ISBN 0-8053-1840-2.
- ^ "It's Your Health". Canadian Government. 2008. http://www.hc-sc.gc.ca/hl-vs/iyh-vsv/food-aliment/sodium-eng.php.
- ^ a b Chapter 3. Foods and Food Components to Reduce, Dietary Guidelines for Americans, 2010, USDA
- ^ "Sodium and Potassium Quick Health Facts". http://health.ltgovernors.com/sodium-and-potassium-health-facts.html. Retrieved 7 November 2011.
- ^ Geleijnse, J. M.; Kok, F. J.; Grobbee, D. E. (2004). "Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations". European journal of public health 14 (3): 235–239. doi:10.1093/eurpub/14.3.235. PMID 15369026.
- ^ Hajjar, I; Kotchen, TA (2003). "Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000". JAMA : the journal of the American Medical Association 290 (2): 199–206. doi:10.1001/jama.290.2.199. PMID 12851274.
- ^ Lawes, C. M.; Vander Hoorn, S; Rodgers, A; International Society of Hypertension (2008). "Global burden of blood-pressure-related disease, 2001". Lancet 371 (9623): 1513–1518. doi:10.1016/S0140-6736(08)60655-8. PMID 18456100. http://www.worldactiononsalt.com/evidence/docs/thelancet_hypertension_05.08.pdf.
- ^ Lloyd-Jones, D.; Adams, R. J.; Brown, T. M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T. B.; Ford, E. et al. (2010). "Executive Summary: Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association". Circulation 121 (7): 948–954. doi:10.1161/CIRCULATIONAHA.109.192666. PMID 20177011.
- ^ "Sodium Lake Explosion 1". Video.google.de. http://video.google.de/videoplay?docid=-2158222101210607510&q=sodium. Retrieved 2010-11-27.
- ^ Girolami, G. S.; Rauchfuss, T. B. and Angelici, R. J., Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA, 1999.ISBN: 0935702482
External links
- The Periodic Table of Videos video of Sodium at YouTube
- Etymology of "natrium" – source of symbol Na
- The Wooden Periodic Table Table's Entry on Sodium
- Dietary Sodium
- Sodium isotopes data from The Berkeley Laboratory Isotopes Project's
Periodic table H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo Alkali metals Alkaline earth metals Lanthanides Actinides Transition metals Other metals Metalloids Other nonmetals Halogens Noble gases Unknown chem. properties Large version Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Alkali metals Lithium
Li
Atomic Number: 3
Atomic Weight: 6.941
Melting Point: 453.85 K
Boiling Point: 1615 K
Specific mass: 0.534 g/cm3
Electronegativity: 0.98Sodium
Na
Atomic Number: 11
Atomic Weight: 22.98976928
Melting Point: 371.15 K
Boiling Point: 1156 K
Specific mass: 0.97 g/cm3
Electronegativity: 0.96Potassium
K
Atomic Number: 19
Atomic Weight: 39.0983
Melting Point: 336.5 K
Boiling Point: 1032 K
Specific mass: 0.86 g/cm3
Electronegativity: 0.82Rubidium
Rb
Atomic Number: 37
Atomic Weight: 85.4678
Melting Point: 312.79 K
Boiling Point: 961 K
Specific mass: 1.53 g/cm3
Electronegativity: 0.82Caesium
Cs
Atomic Number: 55
Atomic Weight: 132.9054519
Melting Point: 301.7 K
Boiling Point: 944 K
Specific mass: 1.93 g/cm3
Electronegativity: 0.79Francium
Fr
Atomic Number: 87
Atomic Weight: 223
Melting Point: 300.15 K
Boiling Point: 950 K
Specific mass: 1.87 g/cm3
Electronegativity: 0.7Categories:- Sodium minerals
- Sodium
- Desiccants
- Dietary minerals
- Reducing agents
- Chemical elements
- Alkali metals
- Biology and pharmacology of chemical elements
- Nuclear reactor coolants
Wikimedia Foundation. 2010.