- (-)-2β-(3-(4-Methylphenyl)isoxazol-5-yl)-3β-(4-chlorophenyl)tropane
-
(-)-2β-(3-(4-Methylphenyl)isoxazol-5-yl)-3β-(4-chlorophenyl)tropane 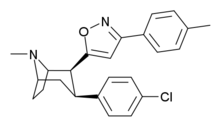
Systematic (IUPAC) name (1R,2S,3S,5S)-8-methyl-2-(3-(4-methylphenyl)isoxazol-5-yl)-3-(4-chlorophenyl)-8-azabicyclo[3.2.1]octane Clinical data Pregnancy cat. ? Legal status ? Identifiers ATC code ? PubChem CID 9800708 Chemical data Formula C24H25ClN2O Mol. mass 392.920 SMILES eMolecules & PubChem RTI-336, (LS-193,309, (-)-2β-(3-(4-methylphenyl)isoxazol-5-yl)-3β-(4-chlorophenyl)tropane) is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine,[1] however it produces relatively mild stimulant effects, with a slow onset and long duration of action.[2] These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse.[3][4] RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration,[5][6] and significantly reduces rates of cocaine use, especially when combined with SSRIs,[7] and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.
See also
- RTI-177
- List of cocaine analogues
References
- ^ Carroll, F.; Pawlush, N.; Kuhar, M.; Pollard, G.; Howard, J. (2004). "Synthesis, monoamine transporter binding properties, and behavioral pharmacology of a series of 3beta-(substituted phenyl)-2beta-(3'-substituted isoxazol-5-yl)tropanes". Journal of Medicinal Chemistry 47 (2): 296–302. doi:10.1021/jm030453p. PMID 14711303.
- ^ Carroll, F.; Fox, B.; Kuhar, M.; Howard, J.; Pollard, G.; Schenk, S. (2006). "Effects of dopamine transporter selective 3-phenyltropane analogs on locomotor activity, drug discrimination, and cocaine self-administration after oral administration". European journal of pharmacology 553 (1–3): 149–156. doi:10.1016/j.ejphar.2006.09.024. PMID 17067572.
- ^ Carroll, F.; Howard, J.; Howell, L.; Fox, B.; Kuhar, M. (2006). "Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse". The AAPS journal 8 (1): E196–E203. doi:10.1208/aapsj080124. PMC 2751440. PMID 16584128. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2751440.
- ^ Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opinion on Emerging Drugs. 2006 Mar;11(1):91-98. doi:10.1517/14728214.11.1.91
- ^ Kimmel, H. .; O'Connor, J. .; Carroll, F. .; Howell, L. . (2007). "Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys". Pharmacology, biochemistry, and behavior 86 (1): 45–54. doi:10.1016/j.pbb.2006.12.006. PMC 1850383. PMID 17258302. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1850383.
- ^ Kimmel, H.; Negus, S.; Wilcox, K.; Ewing, S.; Stehouwer, J.; Goodman, M.; Votaw, J.; Mello, N. et al. (2008). "Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys". Pharmacology, biochemistry, and behavior 90 (3): 453–462. doi:10.1016/j.pbb.2008.03.032. PMC 2453312. PMID 18468667. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2453312.
- ^ Howell, L.; Carroll, F.; Votaw, J.; Goodman, M.; Kimmel, H. (2007). "Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys". The Journal of pharmacology and experimental therapeutics 320 (2): 757–765. doi:10.1124/jpet.106.108324. PMID 17105829.
Phenyltropanes (classifications) 2-Carboxymethyl Esters (3,4-Disubstituted Phenyl)-tropanes Dichloropane • RTI-112 • RTI-353
Arylcarboxy RTI-113 • RTI-120
Carboxyalkyl Acyl β,α Stereochemistry RTI-352 • RTI-274
α,β Stereochemistry Heterocycles: 3-Substituted-isoxazol-5-yl RTI-177 • RTI-336 • RTI-371
Heterocycles: 3-Substituted-1,2,4-oxadiazole RTI-126 • RTI-371
N-alkyl N-replaced (S,O,C) Tropoxane
Irreversible RTI-76
Nortropanes (N-demethylated) Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic amines Dopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugs 
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it.
