- Mesocarb
-
Mesocarb 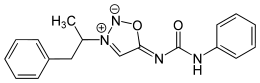
Systematic (IUPAC) name 5-(Phenylcarbamoylimino)-3-(1-phenylpropan-2-yl)-5H-1,2,3-oxadiazol-3-ium-2-ide Clinical data Pregnancy cat. ? Legal status Class C (UK), Schedule III (Can) Routes Oral Pharmacokinetic data Metabolism hepatic Excretion renal Identifiers CAS number 34262-84-5 
ATC code None PubChem CID 71932 ChemSpider 16736988 
UNII UMT8MP2NDU 
Chemical data Formula C18H18N4O2 Mol. mass 322.36 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mesocarb (Sidnocarb, Sydnocarb) is a stimulant drug which was developed in the USSR in the 1970s.[1][2] It has been shown to act as a dopamine reuptake inhibitor[3][4] which is slower acting but longer lasting and less neurotoxic than dextroamphetamine.[5]
Mesocarb is still used for a variety of applications;[6] these include counteracting the sedative effects of benzodiazepine drugs,[7] increasing workload capacity and cardiovascular function,[8] treatment of ADHD and hyperactivity in children,[9][10] as a nootropic,[11] and as a drug to enhance resistance to extremely cold temperatures.[12][13] It is also listed as having antidepressant and anticonvulsant properties.
Mesocarb is sold in Russia as 5 milligram tablets under the brand name Sydnocarb. Hydroxylated metabolites can be detected in urine for up to 10 days after consumption, reflecting a relatively long half-life.[14]
Mesocarb is almost unknown in the western world and is neither used in medicine or studied scientifically to any great extent outside Russia and other countries in the former Soviet Union. It has however been added to the list of drugs under international control and is illegal in most countries, despite its multiple therapeutic applications and the lack of significant abuse potential seen in clinical practice.[15]
Chemistry
Mesocarb is a mesoionic sydnone imine. It has the amphetamine-backbone present, except that the RN has a complicated imine side-chain present.
References
- ^ GB Patent 1262830 - NOVEL SYDNONIMINE DERIVATIVE
- ^ Anokhina IP, Zabrodin GD, Svirinovskiĭ IaE (1974). "[Characteristics of the central action of sidnocarb] [Characteristics of the central action of sidnocarb]" (in Russian). Zh Nevropatol Psikhiatr Im S S Korsakova 74 (4): 594–602. PMID 4825943.
- ^ Anderzhanova EA, Afanas'ev II, Kudrin VS, Rayevsky KS (September 2000). "Effect of d-amphetamine and sydnocarb on the extracellular level of dopamine, 3,4-dihydroxyphenylacetic acid, and hydroxyl radicals generation in rat striatum". Ann N Y Acad Sci. 914: 137–45. doi:10.1111/j.1749-6632.2000.tb05191.x. PMID 11085316. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0077-8923&date=2000&volume=914&spage=137.
- ^ Gainetdinov RR, Sotnikova TD, Grekhova TV, Rayevsky KS (December 1997). "Effects of a psychostimulant drug sydnocarb on rat brain dopaminergic transmission in vivo". Eur J Pharmacol. 340 (1): 53–8. doi:10.1016/S0014-2999(97)01407-6. PMID 9527506. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(97)01407-6.
- ^ Afanas'ev II, Anderzhanova EA, Kudrin VS, Rayevsky KS (2001). "Effects of amphetamine and sydnocarb on dopamine release and free radical generation in rat striatum". Pharmacol Biochem Behav. 69 (3–4): 653–8. doi:10.1016/S0091-3057(01)00574-3. PMID 11509228. http://linkinghub.elsevier.com/retrieve/pii/S0091-3057(01)00574-3.
- ^ Witkin JM, Savtchenko N, Mashkovsky M, et al. (1 March 1999). "Behavioral, toxic, and neurochemical effects of sydnocarb, a novel psychomotor stimulant: comparisons with methamphetamine". J Pharmacol Exp Ther. 288 (3): 1298–310. PMID 10027871. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10027871.
- ^ Valueva LN, Tozhanova NM (1982). "[Sidnocarb correction of the adverse effects of benzodiazepine tranquilizers] [Sidnocarb correction of the adverse effects of benzodiazepine tranquilizers]" (in Russian). Zh Nevropatol Psikhiatr Im S S Korsakova 82 (8): 92–7. PMID 6127851.
- ^ Vinar O, Klein DF, Potter WZ, Gause EM (December 1991). "A survey of psychotropic medications not available in the United States". Neuropsychopharmacology 5 (4): 201–17. PMID 1804161.
- ^ Turova NF, Misionzhnik EIu, Ermolina LA, Aziavchik AV, Krasov VA (1988). "[Excretion of monoamines, their precursors and metabolites in the hyperactivity syndrome in mentally defective children] [Excretion of monoamines, their precursors and metabolites in the hyperactivity syndrome in mentally defective children]" (in Russian). Vopr Med Khim. 34 (1): 47–50. PMID 3369126.
- ^ Krasov VA (1988). "[Sidnocarb treatment of young schoolchildren with the hyperdynamic syndrome] [Sidnocarb treatment of young schoolchildren with the hyperdynamic syndrome]" (in Russian). Zh Nevropatol Psikhiatr Im S S Korsakova 88 (8): 97–101. PMID 3195293.
- ^ Ganiev MM, Kharlamov AN, Raevskiĭ KS, Guseĭnov DIa (October 1987). "[Effect of sidnocarb on learning and memory] [Effect of sidnocarb on learning and memory]" (in Russian). Biull Eksp Biol Med 104 (10): 453–4. PMID 3676468.
- ^ Barer AS, Lakota NG, Ostrovskaia GZ, Shashkov VS (Nov-Dec 1988). "[Pharmacologic correction of the effect of cold on man] [Pharmacologic correction of the effect of cold on man]" (in Russian). Kosm Biol Aviakosm Med 22 (6): 66–73. PMID 2906380.
- ^ Levina MN, Badyshtov BA, Gan'shina TS (2006). "[Thermoprotector properties of a combination of sydnocarb with ladasten] [Thermoprotector properties of a combination of sydnocarb with ladasten]" (in Russian). Eksp Klin Farmakol 69 (1): 71–3. PMID 16579065.
- ^ Shpak AV, Appolonova SA, Semenov VA (January 2005). "Validation of liquid chromatography-electrospray ionization ion trap mass spectrometry method for the determination of mesocarb in human plasma and urine". J Chromatogr Sci 43 (1): 11–21. PMID 15808002. http://openurl.ingenta.com/content/nlm?genre=article&issn=0021-9665&volume=43&issue=1&spage=11&aulast=Shpak.
- ^ Rudenko GM, Altshuler RA (1978). "[Experimental and clinical study of Sydnocarb]" (in Russian). Hung Pharmacotherapy 124: 150–4.
Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic amines Dopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugs Psychostimulants, agents used for ADHD, and nootropics (N06B) Centrally acting sympathomimetics Amphetamine • Amphetaminil • Atomoxetine • Dexmethylphenidate • Dextroamphetamine • Dextromethamphetamine • Fencamfamine • Fenethylline • Lisdexamfetamine • Methylphenidate • Mesocarb • Pemoline • Pipradrol • ProlintaneXanthine derivatives Glutamate receptor CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • S-18986 • Sunifiram • UnifiramEugeroics / Benzhydryl compounds Histamine H3 receptor antagonists GABAA α5 inverse agonists Dopamine D1 receptor agonists α7 nicotinic agonists / PAMs AR-R17779 • PNU-282,987 • SSR-180,711Prolyl endopeptidase inhibitors S-17092Alpha-adrenergic agonists Other psychostimulants and nootropics Acetylcarnitine • Adafenoxate • Bifemelane • Carbenoxolone • Citicoline • Cyprodenate • Ensaculin • Idebenone • Ispronicline • Deanol • Dimebon • Fipexide • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • P7C3 • Pirisudanol • Pyritinol • Rubidium • Sulbutiamine • Taltirelin • Tricyanoaminopropene • Vinpocetine
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it.
