- Bacterial rhodopsins
Pfam_box
Symbol = Bac_rhodopsin
Name =
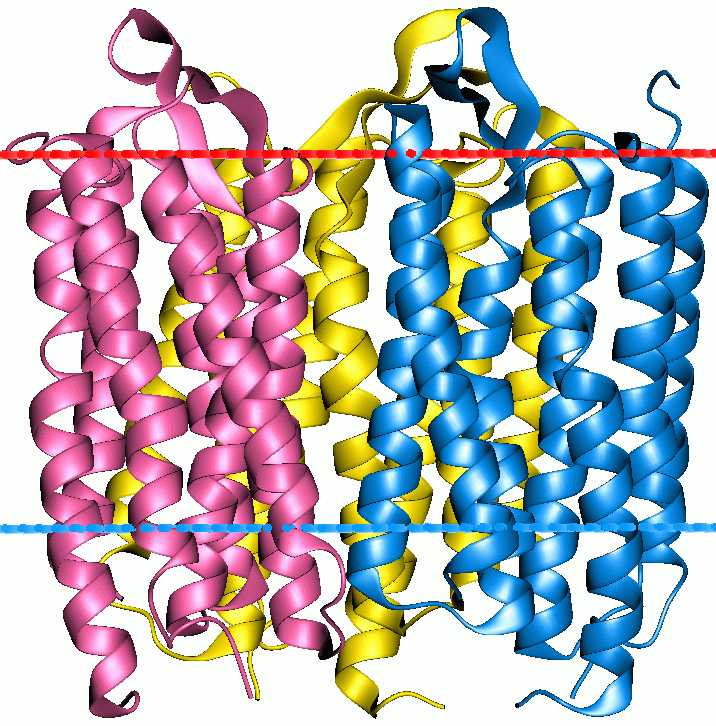
width =
caption =Bacteriorhodopsin trimer
Pfam= PF01036
InterPro= IPR001425
SMART=
PROSITE = PDOC00291
SCOP = 2brd
TCDB = 3.E.1
OPM family= 6
OPM protein= 1vgo
PDB=PDB3|1e12A:29-271 PDB3|1vgoB:19-253 PDB3|1s52B:22-244PDB3|1r84A:22-245 PDB3|1ap9 :22-238 PDB3|1kmeB:22-244PDB3|1r2nA:22-255 PDB3|1pxsA:22-255 PDB3|1p8uA:22-255PDB3|1bac :22-238 PDB3|1brrA:22-253 PDB3|1c3wA:22-244PDB3|1q5iA:22-255 PDB3|1jv6A:22-255 PDB3|1ucqA:22-255PDB3|1iw6A:22-255 PDB3|1x0sA:22-255 PDB3|1tn5A:22-255PDB3|1py6A:22-255 PDB3|1cwqB:22-253 PDB3|1p8hA:22-255PDB3|1tn0B:22-255 PDB3|1s54A:22-244 PDB3|1o0aA:22-255PDB3|1s53A:22-244 PDB3|1pxrA:22-255 PDB3|1f50A:22-244PDB3|1bm1 :22-240 PDB3|1q5jB:22-255 PDB3|1jv7A:22-255PDB3|1e0pB:22-245 PDB3|1s51B:22-244 PDB3|1dzeA:22-255PDB3|1qkpA:22-255 PDB3|2brd :22-241 PDB3|1m0mA:22-255PDB3|1x0k1:22-255 PDB3|1iw9A:22-255 PDB3|1c8sA:22-235PDB3|1c8rA:22-244 PDB3|1mgyA:22-255 PDB3|1fbbA:22-255PDB3|1qkoA:22-255 PDB3|1ixfA:22-255 PDB3|1m0kA:22-255PDB3|1brx :22-241 PDB3|1fbkA:22-255 PDB3|1m0lA:22-255PDB3|1kg9A:22-244 PDB3|1s8lA:22-255 PDB3|1bct :176-244PDB3|1vjmA:22-255 PDB3|1qhjA:22-245 PDB3|1kg8A:22-244PDB3|2at9 :22-240 PDB3|1bad :22-238 PDB3|1bhb :22-83PDB3|1kgbA:22-244 PDB3|1bha :22-83 PDB3|1xjiA:22-255PDB3|1p8iA:22-255 PDB3|1l0mA:22-231 PDB3|1qm8A:22-255PDB3|1x0i1:22-255 PDB3|1s8jA:22-255 PDB3|1f4zA:22-244PDB3|1jgjA:4-217 PDB3|1gueA:4-231 PDB3|1gu8A:4-231PDB3|1h2sA:4-225 PDB3|1xioA:4-233 PDB3|1sr1 :4-214Bacterial rhodopsins are a family of bacterial
opsin s. They are retinal-binding proteins that provide light-dependention transport and sensory functions to a family of halophiliccite journal |author=Oesterhelt D, Tittor J |title=Two pumps, one principle: light-driven ion transport in halobacteria |journal=Trends Biochem. Sci. |volume=14 |issue=2 |pages=57–61 |year=1989 |pmid=2468194 |doi=10.1016/0968-0004(89)90044-3] cite journal |author=Lottspeich F, Oesterhelt D, Blanck A, Ferrando E, Schegk ES |title=Primary structure of sensory rhodopsin I, a prokaryotic photoreceptor |journal=EMBO J. |volume=8 |issue=13 |pages=3963–3971 |year=1989 |pmid=2591367] and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point forretinal (a conserved lysine).The proteins from
halobacteria includebacteriorhodopsin and archaerhodopsin, which are light-drivenproton pump s;halorhodopsin , a light-driven chloride pump; and sensory rhodopsin, which mediates both photoattractant (in the red) andphotophobic (in the ultra-violet) responses. Proteins from other bacteria includeproteorhodopsin .References
Wikimedia Foundation. 2010.
