- Enyne metathesis
An Enyne metathesis is an
organic reaction taking place between analkyne and analkene with ametal carbene catalyst forming abutadiene . This reaction is a variation ofolefin metathesis . ["Enyne Metathesis (Enyne Bond Reorganization)" Diver, S. T.; Giessert, A. J. "Chem. Rev. " 2004, "104(3)", 1317-1382. (Review) (DOI|10.1021/cr020009e)]The general scheme is given by "scheme 1":
:
When the reaction is
intramolecular it is called ring-closing enyne metathesis or RCEYM ("scheme 2")::
with Y representing
oxygen ornitrogen and n an integer.The reaction was first described in 1985 with the conversion of
biphenyl 3.1 to aphenanthrene in "scheme 3": ["Metal-catalyzed rearrangement of alkene-alkynes and the stereochemistry of metallacyclobutene ring opening"Thomas J. Katz and Timothy M. Sivavec "J. Am. Chem. Soc. " 1985, "107(3)", 737 - 738. (DOI|10.1021/ja00289a054)]:
The carbene is a
tungsten carbonyl when used in stoichoimetric amounts (1 equivalent) yields 41% of the phenanthrene 3.2 and when used in catalytic amounts phenanthrene 3.3. Thestereoselectivity of this reaction is large with the metal atom exclusively adding to one of the alkyne carbon atoms in the initial reaction step.Reaction mechanism
The
reaction mechanism for this reaction is outlined in scheme 4::
In the first
catalytic cycle thealkyne group of enyne 4.1 forms a metallacyclobutene intermediate 4.3 with carbene 4.2 with R' and R' ' any organic group required to stabilized it. In the next step the metathesis step is reversed with formation of a new double bond and a new carbenic center in 4.4. The ring-closing step takes place when this center reacts with the alkene group to a metallacyclobutane 4.5 as in a regularolefin metathesis reaction. The butadiene group forms in the last step with expulsion of a new methylene carbene, initiating the next cycle but now with R' = H and R' ' = H.This is the proposed "yne-then-ene" mechanism. Evidence for an "ene-then-yne" pathway is beginning to emerge, especially for ruthenium based catalytic systems.
The driving force for this conversion is the formation of a thermodynamically stable conjugated butadiene.
cope
Enyne metathesis reactions are accelerated by
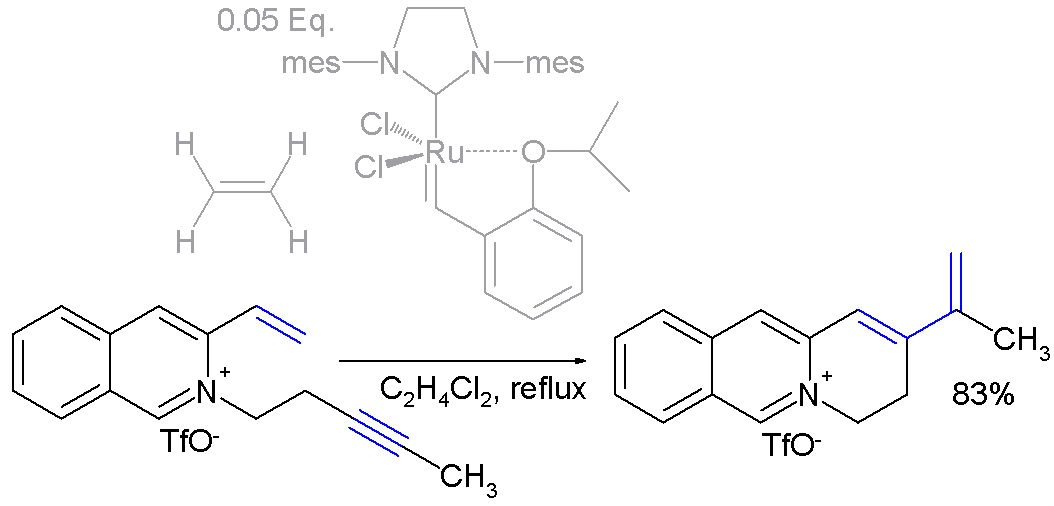
ethylene as is demonstrated in the reaction displayed in "scheme 5": ["Enyne ring-closing metathesis on heteroaromatic cations" Ana Núñez, Ana M. Cuadro, Julio Alvarez-Builla and Juan J. Vaquero "Chem. Commun. ", 2006, 2690-2692. (DOI|10.1039/b602420c)]
400px|Scheme 5. Enyne metathesis synthesis of 2-vinyl-substituted3,4-dihydroquinolizinium saltsIn this reaction with the
Hoveyda-Grubbs Catalyst , ethylene converts the alkyne group to the correspondingdiene group prior to the reaction with the alkene group.References
Wikimedia Foundation. 2010.