Niementowski quinazoline synthesis
- Niementowski quinazoline synthesis
-
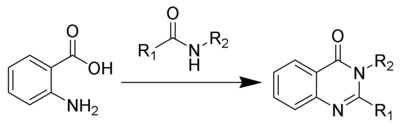
The Niementowski quinazoline synthesis is the chemical reaction of anthranilic acids with amides to form 4-oxo-3,4-dihydroquinazolines.[1][2][3]
References
- ^ Stefan Niementowski, v. J. Prakt. Chem. 1895, 51, 564.
- ^ Williamson, T. A. Heterocyclic Compounds 1957, 6, 331. (Review)
- ^ Cuny, E. et al. Tetrahedron Lett. 1980, 21, 3029. (Review)
See also
Categories: - Condensation reactions
- Heterocycle forming reactions
- Name reactions
Wikimedia Foundation.
2010.
Look at other dictionaries:
Niementowski quinoline synthesis — The Niementowski quinoline synthesis is the chemical reaction of anthranilic acids and ketones (or aldehydes) to form γ hydroxyquinoline derivatives.[1][2][3][4] … Wikipedia
Quinazoline — chembox new ImageFile=Quinazoline numbering.png ImageSize=120px IUPACName=quinazoline OtherNames= Section1=Chembox Identifiers CASNo=253 82 7 PubChem=9210 SMILES=C1=CC=C2C(=C1)C=NC=N2 Section2=Chembox Properties Formula=C8H6N2 MolarMass=130.15… … Wikipedia
Stefan Niementowski — Infobox Scientist name = Stefan Niementowski image width = caption = Stefan Niementowski birth date = birth date|1866|8|4 birth place = Zhovkva, Poland now Ukraine residence = nationality = Polish death date = death date and… … Wikipedia
Síntesis de quinazolina de Niementowski — La síntesis de quinazolinas de Niementowski es un método de síntesis orgánica en donde el ácido antranílico reacciona con amidas para formar 4 oxo 3,4 dihidroquinazolinas.>[1] [2] [3] … Wikipedia Español
Ниментовский, Стефан — Стефан Доминик Ниментовский Stefan Dominik Niementowski … Википедия
List of organic reactions — Well known reactions and reagents in organic chemistry include Contents: A B C D E F G H I J K L M N O P Q R S T U V W X Y Z See also Ext … Wikipedia
Pfitzinger reaction — The Pfitzinger reaction (also known as the Pfitzinger Borsche reaction) is the chemical reaction of isatin with base and a carbonyl compound to give substituted quinoline 4 carboxylic acids. [Pfitzinger, W. J. Prakt. Chem. 1886, 33 , 100.]… … Wikipedia