- Michaelis–Arbuzov reaction
-
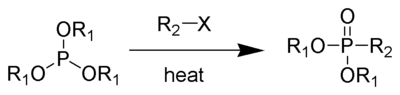
The Michaelis–Arbuzov reaction (also called the Arbuzov reaction) is the chemical reaction of a trialkyl phosphite and an alkyl halide to form a phosphonate.
The reaction was discovered by August Michaelis in 1898[1], and greatly explored by Aleksandr Arbuzov soon thereafter.[2][3] This reaction is widely used for the synthesis of various phosphonates, phosphinates, and phosphine oxides. Several reviews have been published.[4][5]
Contents
Reaction mechanism
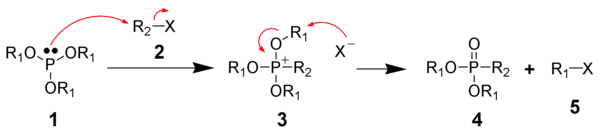
The Michaelis–Arbuzov reaction is initiated with the SN2 reaction of the nucleophilic phosphite (1) with the electrophilic alkyl halide (2) to give a phosphonium intermediate (3). Triaryl phosphites, which are unable to perform the second step of the Michaelis-Arbuzov reaction, have been shown to produce stable phosphonium salts.[6] Likewise, aryl and vinyl halides are less reactive towards phosphites.
The displaced halide anion reacts via another SN2 reaction with the phosphonium intermediate to give the desired phosphonate (4) and another alkyl halide (5). When chiral phosphonium intermediates are produced, it has been shown the halide substitution proceeds with inversion of configuration, as expected by a SN2 reaction.[7]
As a general guideline, the reactivity of the organic halide (2) can be listed as follows: (from most reactive to least reactive)
- RCOX > RCH2X > RR'CHX >> RR'R"CX
and
- RI > RBr > RCl
The reaction of α-bromo- and α-chloroketones with phosphites yields a vinyl phosphate instead of an alkyl phosphonate – the Perkow reaction. α-Iodoketones do, in fact, give the a phosphonate.[8] Other methods of producing β-ketophosphonates have been developed.[9]
References
- ^ Michaelis, A.; Kaehne, R. (1898). "Ueber das Verhalten der Jodalkyle gegen die sogen. Phosphorigsäureester oder O-Phosphine". Berichte 31: 1048. doi:10.1002/cber.189803101190.
- ^ Arbuzov, A. E. (1906). J. Russ. Phys. Chem. Soc. 38: 687.
- ^ Arbuzov, A. E. (1906). Chem. Zentr. II: 1639.
- ^ Arbuzov, B. A. (1964). "Michaelis–Arbusow- und Perkow-Reaktionen". Pure Appl. Chem. 9 (2): 307–353. doi:10.1351/pac196409020307.
- ^ Bhattacharya, A. K.; Thyagarajan, G. (1981). "Michaelis–Arbuzov rearrangement". Chem. Rev. 81 (4): 415–430. doi:10.1021/cr00044a004.
- ^ Landuer, S. R.; Rydon, H. N. (1953). "458. The organic chemistry of phosphorus. Part I. Some new methods for the preparation of alkyl halides". J. Chem. Soc.: 2224. doi:10.1039/jr9530002224.
- ^ Gerrard, W.; Green, W. J. (1951). "568. Mechanism of the formation of dialkyl alkylphosphonates". J. Chem. Soc.: 2550. doi:10.1039/jr9510002550.
- ^ Jacobsen, H. I.; Griffin, M. J.; Preis, S.; Jensen, E. V. (1957). "Phosphonic Acids. IV. Preparation and Reactions of β-Ketophosphonate and Enol Phosphate Esters". J. Am. Chem. Soc. 79 (10): 2608. doi:10.1021/ja01567a067.
- ^ Nagata, W.; Wakabayashi, T.; Hayase, Y. (1988), "Diethyl 2-(cyclohexylamino)vinylphosphonate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0448; Coll. Vol. 6: 448
See also
External links
- Ford-Moore, A. H.; Perry, B. J. Organic Syntheses, Coll. Vol. 4, p.325 (1963); Vol. 31, p.33 (1951). (Article)
- Davidsen, S. K.; Phllips, G. W.; Martin, S. F. Organic Syntheses, Coll. Vol. 8, p.451 (1993); Vol. 65, p.119 (1987). (Article)
- Enders, D.; von Berg, S.; Jandeleit, B. Organic Syntheses, Coll. Vol. 10, p.289 (2004); Vol. 78, p.169 (2002). (Article)
Categories:- Substitution reactions
- Name reactions
Wikimedia Foundation. 2010.