- Pyrosulfate
-
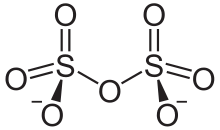
In chemistry, disulfate or pyrosulfate is the anion with the molecular formula [S2O7]2−. Disulfate is the IUPAC name. [1] It has a dichromate like structure and can be visualised as two corner sharing SO4 tetrahedra, with a bridging oxygen atom.[2] In this compound sulfur has an oxidation state of +6. Disulfate is the conjugate base of the hydrogen disulfate (hydrogen pyrosulfate) ion HS2O7-, which in turn is the conjungate base of disulfuric acid (pyrosulfuric acid).
See also
References
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- ^ The crystal structure determination and refinements of K2S2O7, KNaS2O7 and Na2S2O7 from X-ray powder and single crystal diffraction data Ståhl K , Balic-Zunic T, da Silva F, Eriksen K M , Berg R W Fehrmann R Journal of Solid State Chemistry 178, 1697-1704, (2005) doi:10.1016/j.jssc.2005.03.022
Categories:- Pyrosulfates
Wikimedia Foundation. 2010.