- Conotoxin
-
Alpha conotoxin precursor 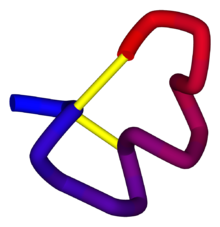
α-Conotoxin PnIB from C. pennaceus, disulfide bonds shown in yellow. From the University of Michigan's Orientations of Proteins in Membranes database, PDB 1AKG. Identifiers Symbol Toxin_8 Pfam PF07365 InterPro IPR009958 PROSITE PDOC60004 SCOP 1mii OPM family 157 OPM protein 1akg Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Omega conotoxin 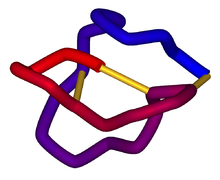
Schematic diagram of the three-dimensional structure of ω-conotoxin MVIIA (ziconotide). Disulfide bonds are shown in gold. From PDB 1DW5. Identifiers Symbol Conotoxin Pfam PF02950 InterPro IPR004214 SCOP 2cco OPM family 120 OPM protein 1fyg Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.
Conotoxins, which are peptides consisting of 10 to 30 amino acid residues, typically have one or more disulfide bonds. Conotoxins have a variety of mechanisms of actions, most of which have not been determined. However it appears that many of these peptides modulate the activity of ion channels.[1] Over the last few decades conotoxins have been subject of pharmacological interest.[2]
Contents
Types and biological activities of conotoxins
The number of conotoxins whose activities have been determined so far is five, and they are called the α(alpha)-, δ(delta)-, κ(kappa)-, μ(mu)-, and ω(omega)- types. Each of the five types of conotoxins attacks a different target:
- α-conotoxin inhibits nicotinic acetylcholine receptors at nerves and muscles.[3]
- δ-conotoxin inhibits the inactivation of voltage-dependent sodium channels.[4]
- κ-conotoxin inhibits potassium channels.[5]
- μ-conotoxin inhibits voltage-dependent sodium channels in muscles.[6]
- ω-conotoxin inhibits N-type voltage-dependent calcium channels.[7] Because N-type voltage-dependent calcium channels are related to algesia (sensitivity to pain) in the nervous system, ω-conotoxin has an analgesic effect: the effect of ω-conotoxin M VII A is 100 to 1000 times that of morphine.[8] Therefore a synthetic version of ω-conotoxin M VII A has found application as an analgesic drug ziconotide (Prialt).[9]
Disulfide connectivities
Types of conotoxins also differ in the number and pattern of disulfide bonds.[10] The disulfide bonding network, as well as specific amino acids in inter-cysteine loops, provide the specificity of conotoxins.[11]
Omega, delta and kappa conotoxins
Omega, delta and kappa families of conotoxins have a knottin or inhibitor cysteine knot scaffold. The knottin scaffold is a very special disulfide-through-disulfide knot, in which the III-VI disulfide bond crosses the macrocycle formed by two other disulfide bonds (I-IV and II-V) and the interconnecting backbone segments, where I-VI indicates the six cysteine residues starting from the N-terminus. The cysteine arrangements are the same for omega, delta and kappa families, even though omega conotoxins are calcium channel blockers, whereas delta conotoxins delay the inactivation of sodium channels, and kappa conotoxins are potassium channel blockers.[10]
Mu conotoxins
Mu-conotoxin 
nmr solution structure of piiia toxin, nmr, 20 structures Identifiers Symbol Mu-conotoxin Pfam PF05374 Pfam clan CL0083 InterPro IPR008036 SCOP 1gib Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Mu-conotoxins have two types of cysteine arrangements, but the knottin scaffold is not observed.[12] Mu-conotoxins target the muscle-specific voltage-gated sodium channels,[10] and are useful probes for investigating voltage-dependent sodium channels of excitable tissues.[12][13] Mu-conotoxins target the voltage-gated sodium channels, preferentially those of skeletal muscle,[14] and are useful probes for investigating voltage-dependent sodium channels of excitable tissues.[15]
Different subtypes of voltage-gated sodium channels are found in different tissues in mammals, eg., in muscle and brain, and studies have been carried out to determine the sensitivity and specificity of the mu-conotoxins for the different isoforms.[16]
Alpha conotoxins
Alpha conotoxins have two types of cysteine arrangements,[17] and are competitive nicotinic acetylcholine receptor antagonists.
See also
References
- ^ Terlau H, Olivera BM (2004). "Conus venoms: a rich source of novel ion channel-targeted peptides". Physiol. Rev. 84 (1): 41–68. doi:10.1152/physrev.00020.2003. PMID 14715910.
- ^ Olivera BM, Teichert RW (2007). "Diversity of the neurotoxic Conus peptides: a model for concerted pharmacological discovery.". Mol Interv 7 (5): 251–60. doi:10.1124/mi.7.5.7. PMID 17932414. http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17932414.
- ^ Nicke A, Wonnacott S, Lewis RJ (2004). "Alpha-conotoxins as tools for the elucidation of structure and function of neuronal nicotinic acetylcholine receptor subtypes". Eur. J. Biochem. 271 (12): 2305–2319. doi:10.1111/j.1432-1033.2004.04145.x. PMID 15182346.
- ^ Leipold E, Hansel A, Olivera BM, Terlau H, Heinemann SH (2005). "Molecular interaction of delta-conotoxins with voltage-gated sodium channels". FEBS Lett. 579 (18): 3881–3884. doi:10.1016/j.febslet.2005.05.077. PMID 15990094.
- ^ Shon KJ, Stocker M, Terlau H, Stühmer W, Jacobsen R, Walker C, Grilley M, Watkins M, Hillyard DR, Gray WR, Olivera BM (1998). "kappa-Conotoxin PVIIA is a peptide inhibiting the shaker K+ channel". J. Biol. Chem. 273 (1): 33–38. doi:10.1074/jbc.273.1.33. PMID 9417043.
- ^ Li RA, Tomaselli GF (2004). "Using the deadly mu-conotoxins as probes of voltage-gated sodium channels". Toxicon 44 (2): 117–122. doi:10.1016/j.toxicon.2004.03.028. PMC 2698010. PMID 15246758. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2698010.
- ^ Nielsen KJ, Schroeder T, Lewis R (2000). "Structure-activity relationships of omega-conotoxins at N-type voltage-sensitive calcium channels" (abstract). J. Mol. Recognit. 13 (2): 55–70. doi:10.1002/(SICI)1099-1352(200003/04)13:2<55::AID-JMR488>3.0.CO;2-O. PMID 10822250. http://www3.interscience.wiley.com/cgi-bin/abstract/72502378/ABSTRACT.
- ^ Bowersox SS, Luther R (1998). "Pharmacotherapeutic potential of omega-conotoxin MVIIA (SNX-111), an N-type neuronal calcium channel blocker found in the venom of Conus magus". Toxicon 36 (11): 1651–1658. doi:10.1016/S0041-0101(98)00158-5. PMID 9792182.
- ^ Prommer E (2006). "Ziconotide: a new option for refractory pain". Drugs Today 42 (6): 369–78. doi:10.1358/dot.2006.42.6.973534. PMID 16845440.
- ^ a b c Jones RM, McIntosh JM (2001). "Cone venom--from accidental stings to deliberate injection". Toxicon 39 (10): 1447–1451. doi:10.1016/S0041-0101(01)00145-3. PMID 11478951.
- ^ Sato K, Kini RM, Gopalakrishnakone P, Balaji RA, Ohtake A, Seow KT, Bay BH (2000). "lambda-conotoxins, a new family of conotoxins with unique disulfide pattern and protein folding. Isolation and characterization from the venom of Conus marmoreus". J. Biol. Chem. 275 (50): 39516–39522. doi:10.1074/jbc.M006354200. PMID 10988292.
- ^ a b Nielsen KJ, Watson M, Adams DJ, Hammarström AK, Gage PW, Hill JM, Craik DJ, Thomas L, Adams D, Alewood PF, Lewis RJ (July 2002). J. Biol. Chem. 277 (30): 27247–55. doi:10.1074/jbc.M201611200. PMID 12006587.
- ^ Zeikus RD, Gray WR, Cruz LJ, Olivera BM, Kerr L, Moczydlowski E, Yoshikami D (1985). "Conus geographus toxins that discriminate between neuronal and muscle sodium channels". J. Biol. Chem. 260 (16): 9280–8. PMID 2410412.
- ^ McIntosh JM, Jones RM (October 2001). "Cone venom--from accidental stings to deliberate injection". Toxicon 39 (10): 1447–51. doi:10.1016/S0041-0101(01)00145-3. PMID 11478951.
- ^ Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E (August 1985). "Conus geographus toxins that discriminate between neuronal and muscle sodium channels". J. Biol. Chem. 260 (16): 9280–8. PMID 2410412.
- ^ Floresca CZ (2003). "A comparison of the mu-conotoxins by [3Hsaxitoxin binding assays in neuronal and skeletal muscle sodium channel."]. Toxicol Appl Pharmacol 190 (2): 95–101. PMID 12878039. http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12878039.
- ^ Gray WR, Olivera BM, Zafaralla GC, Ramilo CA, Yoshikami D, Nadasdi L, Hammerland LG, Kristipati R, Ramachandran J, Miljanich G (1992). "Novel alpha- and omega-conotoxins from Conus striatus venom". Biochemistry 31 (41): 11864–11873. doi:10.1021/bi00162a027. PMID 1390774.
This article includes text from the public domain Pfam and InterPro IPR008036External links
- MeSH Conotoxins
- Baldomero "Toto" Olivera. "iBioSeminar on Conus Peptides". The American Society for Cell Biology. http://www.ibioseminars.org/lectures/chemicalbiologybiophysics/baldomero-olivera.html.
- Kaas Q, Westermann JC, Halai R, Wang CK, Craik DJ. "ConoServer". Institute of Molecular Bioscience, The University of Queensland, Australia. http://www.conoserver.org/. Retrieved 2009-06-02. "A database for conopeptide sequences and structures"
General All-α folds: All-β folds: α/β folds: α+β folds: Irregular folds: Conotoxin←Secondary structureCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeThis article includes text from the public domain Pfam and InterPro IPR004214
Categories:- Neurotoxins
- Peripheral membrane proteins
- Ion channel toxins
- Invertebrate toxins
Wikimedia Foundation. 2010.
Look at other dictionaries:
Conotoxin — Als Conotoxine bezeichnet man eine Gruppe von Nervengiften, die aus Meeresschnecken, vor allem der Kegelschnecken Gattung Conus, stammen, die aber auch synthetisch hergestellt werden können. Die meisten Conotoxine sind kurze Peptide und bestehen… … Deutsch Wikipedia
Ziconotid — Omega Conotoxin MVIIA (Conus magus) Proteinrückgrat, Disulfidbrücken gelb, nach … Deutsch Wikipedia
Conus victoriae — Scientific classification Kingdom: Animalia Phylum: Mollusca Class: Gastro … Wikipedia
Nicotinic acetylcholine receptor — Acetylcholine Nicotine … Wikipedia
Lourdes J. Cruz — is a biochemist whose research has contributed to the understanding of the biochemistry of toxic peptides from the venom of fish hunting Conus marine snails. The characterization of over 50 biologically active peptides from the snail s venom had… … Wikipedia
Phencyclidine — Systematic (IUPAC) name … Wikipedia
Botulinum toxin — Clinical data Pregnancy cat. ? Legal status ? (US) Rout … Wikipedia
Tetrodotoxin — Chembox new ImageFile=Tetrodotoxin 2D skeletal.png ImageSize= ImageFile2=Tetrodotoxin 3D balls.png IUPACName=Octahydro 12 (hydroxymethyl) 2 imino 5,9:7,10a dimethano 10aH [1,3] dioxocino [6,5 d] pyrimidine 4,7,10,11,12 pentol… … Wikipedia
Neurotoxin — A neurotoxin is a toxin that acts specifically on nerve cells[1] (neurons), usually by interacting with membrane proteins such as ion channels. Some sources are more general, and define the effect of neurotoxins as occurring at nerve tissue.[2]… … Wikipedia
Dizocilpine — Systematic (IUPAC) name (+) 5 methyl 10,11 dihydro 5H dibenzo[a,d]cyclohepten 5,10 imine maleate Clinical data Pregnancy cat … Wikipedia
