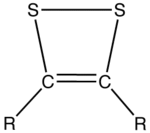
- Dithiete
-
Dithietes are unsaturated heterocyclic compounds that contain two sulfur atoms and two sp2-hybridized carbon centers. Having 6 π electrons, dithietes are aromatic. Two isomers are possible for this small class of organosulfur compounds, 1,2-dithietes, where the sulfur atoms are adjacent, and 1,3-dithietes, where the sulfur atoms are not adjacent. 1,3-Dithietes have not been isolated but a few 1,2-dithietes are known.[1] Unsubstituted 1,2-Dithiete has been generated in thermolytic reactions and was characterized by microwave spectroscopy, UV-photoelectron spectroscopy and IR spectroscopy in low temperature matrix. The open ring structure, dithioglyoxal is less stable than 1,2-dithiete.[2][3]
Quantum chemical calculations can reproduce the observed higher stability of 1,2-dithiete only if large basis-sets with polarization functions are used.[4][5]
However, low temperature photolysis of 1,3-dithiol-2-one in solid argon or nitrogen matrix produces trans-dithioglyoxal.[6]
References
- ^ J. P. Donahue; R. H. Holm (1998). "3,4-Bis(1-adamantyl)-1,2-dithiete: the First Structurally Characterized Dithiete Unsupported by a Ring or Benzenoid Frame". Acta Crystallographica C54 (8): 1175–1178. doi:10.1107/S0108270198002935.
- ^ Reinhard Schulz; Armin Schweig; Klaus Hartke; Joachim Koester (1983). "Theory and application of photoelectron spectroscopy. 100. Variable-temperature photoelectron spectral study of 1,3-dithiol-2-one and 4,5-disubstituted 1,3-dithiol-2-ones. Thermal generation of 1,2-dithiete, 3,4-disubstituted 1,2-dithietes, and dialkyl tetrathiooxalates". Journal of the American Chemical Society 105 (14): 4519–4528. doi:10.1021/ja00352a004.
- ^ Frank Diehl; Hermann Meyer; Armin Schweig; B. Andes Hess Jr.; Juergen Fabian (September 1989). "1,2-Dithiete is more stable than 1,2-dithioglyoxal as evidenced by a combined experimental and theoretical IR spectroscopic approach". J. Amer. Chem. Soc. 111 (19): 7651–7653. doi:10.1021/ja00201a076. http://pubs.acs.org/doi/abs/10.1021/ja00201a076. Retrieved 2009-11-10.
- ^ Jonas V.; Frenking G. (1991). "On the crucial importance of polarization functions for the calculation of molecules with third-row elements: the conformations of chlorocarbonyl isocyanate ClC (O) NCO and the equilibrium of 1,2-dithioglyoxal with its cyclic isomer 1,2-dithiete". Chemical Physics Letters 177 (2): 175–183. doi:10.1016/0009-2614(91)90064-G.
- ^ Gonzalez L.; Mo O.; Yanez M. (13 December 1996). "High-level ab initio calculations on the 1,2-dithioglyoxal/1,2-dithiete isomerism". Chemical Physics Letters 263 (3): 407–413(7). Bibcode 1996CPL...263..407G. doi:10.1016/S0009-2614(96)01240-7.
- ^ Małgorzata Muchaa; Magdalena Pagacza; Zofia Mielke (6 June 2008). "Infrared detection of dithioglyoxal from photolysis of 1,3-dithiol-2-one in solid argon and nitrogen". Chemical Physics Letters 458 (1–3): 39–43. doi:10.1016/j.cplett.2008.04.088.
Categories:- Organosulfur compounds
- Organic disulfides
- Sulfur heterocycles
Wikimedia Foundation. 2010.