- Cyclohexenone
-
Cyclohexenone[1] 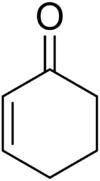 1-Cyclohex-2-enone
1-Cyclohex-2-enoneIdentifiers CAS number 930-68-7 
PubChem 13594 ChemSpider 13005 
KEGG C02395 
ChEBI CHEBI:15977 
Jmol-3D images Image 1 - C1CC=CC(=O)C1
Properties Molecular formula C6H8O Molar mass 96.12712 Appearance Clear colorless liquid Density 0.993 g/mL Melting point −53 °C
Boiling point 171-173 °C
Hazards NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances.[2] It is a clear colorless liquid with a boiling point of 171-173 °C.
Industrially, cyclohexenone is prepared from phenol by Birch reduction.[3]
Common reactions involving cyclohexenone include nucleophilic conjugate addition with organocopper reagents, Michael reactions and Robinson annulations.[4][5]
References
- ^ Cyclohexenone at Sigma-Aldrich
- ^ Podraze, K.F. Org. Prep. Proced. Int., 1991, 23, p. 217.
- ^ Organic Building Blocks of the Chemical Industry, Harry H. Szmant, ISBN 978-0471855453
- ^ Michael G. Organ and Paul Anderson (1996). "Carbonyl and Conjugate Additions to Cyclohexenone: Experiments Illustrating Reagent Selectivity". Journal of Chemical Education 73 (12): 1193. doi:10.1021/ed073p1193.
- ^ Tet. Lett. 34, 3881, (1993)
Categories:- Ketones
- Reagents for organic chemistry
Wikimedia Foundation. 2010.

