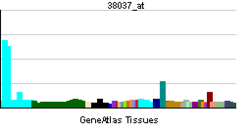
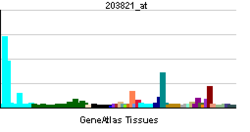
- Heparin-binding EGF-like growth factor
-
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of proteins. It has been shown to play a role in wound healing, cardiac hypertrophy and heart development and function.[1]
Contents
Interactions
Heparin-binding EGF-like growth factor has been shown to interact with NRD1,[2] Zinc finger and BTB domain-containing protein 16[3][4] and BAG1.[5]
References
- ^ Nanba D. and Higashiyama S. (2004). "Dual intracellular signaling by proteolytic cleavage of membrane-anchored heparin-binding EGF-like growth factor". Cytokine Growth Factor Rev. 15 (1): 13–19. doi:10.1016/j.cytogfr.2003.10.002. PMID 14746810.
- ^ Nishi, E; Prat A, Hospital V, Elenius K, Klagsbrun M (Jul. 2001). "N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration". EMBO J. (England) 20 (13): 3342–50. doi:10.1093/emboj/20.13.3342. ISSN 0261-4189. PMC 125525. PMID 11432822. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=125525.
- ^ Nanba, Daisuke; Mammoto Akiko, Hashimoto Koji, Higashiyama Shigeki (Nov. 2003). "Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF". J. Cell Biol. (United States) 163 (3): 489–502. doi:10.1083/jcb.200303017. ISSN 0021-9525. PMC 2173632. PMID 14597771. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2173632.
- ^ Nanba, Daisuke; Toki Fujio, Higashiyama Shigeki (Jul. 2004). "Roles of charged amino acid residues in the cytoplasmic domain of proHB-EGF". Biochem. Biophys. Res. Commun. (United States) 320 (2): 376–82. doi:10.1016/j.bbrc.2004.05.176. ISSN 0006-291X. PMID 15219838.
- ^ Lin, J; Hutchinson L, Gaston S M, Raab G, Freeman M R (Aug. 2001). "BAG-1 is a novel cytoplasmic binding partner of the membrane form of heparin-binding EGF-like growth factor: a unique role for proHB-EGF in cell survival regulation". J. Biol. Chem. (United States) 276 (32): 30127–32. doi:10.1074/jbc.M010237200. ISSN 0021-9258. PMID 11340068.
Further reading
- Higashiyama S, Lau K, Besner GE, et al. (1992). "Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein". J. Biol. Chem. 267 (9): 6205–12. PMID 1556128.
- Yoshizumi M, Kourembanas S, Temizer DH, et al. (1992). "Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells". J. Biol. Chem. 267 (14): 9467–9. PMID 1577791.
- Higashiyama S, Abraham JA, Miller J, et al. (1991). "A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF". Science 251 (4996): 936–9. doi:10.1126/science.1840698. PMID 1840698.
- Iwamoto R, Senoh H, Okada Y, et al. (1991). "An antibody that inhibits the binding of diphtheria toxin to cells revealed the association of a 27-kDa membrane protein with the diphtheria toxin receptor". J. Biol. Chem. 266 (30): 20463–9. PMID 1939101.
- Hayes H, Kaneda Y, Uchida T, Okada Y (1988). "Regional assignment of the gene for diphtheria toxin sensitivity using subchromosomal fragments in microcell hybrids". Chromosoma 96 (1): 26–32. doi:10.1007/BF00285879. PMID 3436221.
- Bennett KL, Jackson DG, Simon JC, et al. (1995). "CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor". J. Cell Biol. 128 (4): 687–98. doi:10.1083/jcb.128.4.687. PMC 2199889. PMID 7532176. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2199889.
- Pathak BG, Gilbert DJ, Harrison CA, et al. (1995). "Mouse chromosomal location of three EGF receptor ligands: amphiregulin (Areg), betacellulin (Btc), and heparin-binding EGF (Hegfl)". Genomics 28 (1): 116–8. doi:10.1006/geno.1995.1116. PMID 7590736.
- Mitamura T, Higashiyama S, Taniguchi N, et al. (1995). "Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity". J. Biol. Chem. 270 (3): 1015–9. doi:10.1074/jbc.270.3.1015. PMID 7836353.
- Hashimoto K, Higashiyama S, Asada H, et al. (1994). "Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes". J. Biol. Chem. 269 (31): 20060–6. PMID 8051092.
- Kobrin MS, Funatomi H, Friess H, et al. (1994). "Induction and expression of heparin-binding EGF-like growth factor in human pancreatic cancer". Biochem. Biophys. Res. Commun. 202 (3): 1705–9. doi:10.1006/bbrc.1994.2131. PMID 8060360.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Thompson SA, Higashiyama S, Wood K, et al. (1994). "Characterization of sequences within heparin-binding EGF-like growth factor that mediate interaction with heparin". J. Biol. Chem. 269 (4): 2541–9. PMID 8300582.
- Fen Z, Dhadly MS, Yoshizumi M, et al. (1993). "Structural organization and chromosomal assignment of the gene encoding the human heparin-binding epidermal growth factor-like growth factor/diphtheria toxin receptor". Biochemistry 32 (31): 7932–8. doi:10.1021/bi00082a014. PMID 8347598.
- Elenius K, Paul S, Allison G, et al. (1997). "Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation". EMBO J. 16 (6): 1268–78. doi:10.1093/emboj/16.6.1268. PMC 1169725. PMID 9135143. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1169725.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Louie GV, Yang W, Bowman ME, Choe S (1998). "Crystal structure of the complex of diphtheria toxin with an extracellular fragment of its receptor". Mol. Cell 1 (1): 67–78. doi:10.1016/S1097-2765(00)80008-8. PMID 9659904.
- Borer JG, Park JM, Atala A, et al. (1999). "Heparin-binding EGF-like growth factor expression increases selectively in bladder smooth muscle in response to lower urinary tract obstruction". Lab. Invest. 79 (11): 1335–45. PMID 10576204.
- Nakamura K, Mitamura T, Takahashi T, et al. (2000). "Importance of the major extracellular domain of CD9 and the epidermal growth factor (EGF)-like domain of heparin-binding EGF-like growth factor for up-regulation of binding and activity". J. Biol. Chem. 275 (24): 18284–90. doi:10.1074/jbc.M907971199. PMID 10749879.
- Duque JL, Adam RM, Mullen JS, et al. (2001). "Heparin-binding epidermal growth factor-like growth factor is an autocrine mediator of human prostate stromal cell growth in vitro". J. Urol. 165 (1): 284–8. doi:10.1097/00005392-200101000-00080. PMID 11125426.
- Lin J, Hutchinson L, Gaston SM, et al. (2001). "BAG-1 is a novel cytoplasmic binding partner of the membrane form of heparin-binding EGF-like growth factor: a unique role for proHB-EGF in cell survival regulation". J. Biol. Chem. 276 (32): 30127–32. doi:10.1074/jbc.M010237200. PMID 11340068.
PDB gallery External links
Growth factors Fibroblast EGF-like domain TGFβ pathway Insulin-like Platelet-derived Vascular endothelial Other Categories:- Human proteins
- Growth factors
- Morphogens
- Chromosome 5 gene stubs
- Cell biology stubs
Wikimedia Foundation. 2010.