- Temafloxacin
-
Temafloxacin 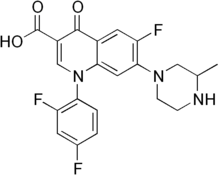
Systematic (IUPAC) name 1-(2,4-Difluorophenyl)-6-fluoro-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid Clinical data Pregnancy cat. ? Legal status Withdrawn Routes Oral Identifiers ATC code J01MA05 PubChem CID 60021 DrugBank DB01405 ChemSpider 54143 
UNII 1WZ12GTT67 
KEGG D02469 
ChEMBL CHEMBL277100 
Chemical data Formula C21H18F3N3O3 Mol. mass 417.381 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Temafloxacin (marketed by Abbott Laboratories as Omniflox), is a fluoroquinolone antibiotic drug which was withdrawn from sale in the United States shortly after its approval in 1992 because of serious adverse effects resulting in three deaths.[citation needed]
Omniflox was approved to treat lower respiratory tract infections, genital and urinary infections like prostatitis, and skin infections in the United States by the Food and Drug Administration in January 1992. Severe adverse reactions, including allergic reactions and hemolytic anemia,[1] developed in about fifty patients during the first four months of its use, leading to three patient deaths. Abbott withdrew the drug from sale in June 1992.
See also
- Fluoroquinolone toxicity
- Fluoroquinolone
References
- ^ Rubinstein, E. (2001). "History of quinolones and their side effects.". Chemotherapy 47 Suppl 3: 3–8; discussion 44–8. doi:10.1159/000057838. PMID 11549783.
External links
- FDA press release June 5, 1992.[dead link]
Antibacterials: nucleic acid inhibitors (J01E, J01M) Antifolates
(inhibits
purine metabolism,
thereby inhibiting
DNA and RNA synthesis)Sulfonamides
(DHPS inhibitor)Other/ungroupedCombinationsTopoisomerase
inhibitors/
quinolones/
(inhibits
DNA replication)1st g.2nd g.Ciprofloxacin# • Enoxacin‡ • Fleroxacin‡ • Lomefloxacin • Nadifloxacin • Ofloxacin • Norfloxacin • Pefloxacin • Rufloxacin3rd g.Balofloxacin • Grepafloxacin‡ • Levofloxacin • Pazufloxacin • Sparfloxacin‡ • Temafloxacin‡ • Tosufloxacin4th g.Besifloxacin • Clinafloxacin† • Garenoxacin • Gemifloxacin • Moxifloxacin • Gatifloxacin‡ • Sitafloxacin • Trovafloxacin‡/Alatrofloxacin‡ • PrulifloxacinVet.Related (DG)Anaerobic DNA
inhibitorsNitrofuran derivativesRNA synthesis 
This systemic antibacterial-related article is a stub. You can help Wikipedia by expanding it.
