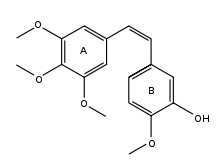
- Combretastatin
-
Combretastatin 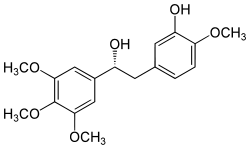 5-[(2R)-2-hydroxy-2-(3,4,5-trimethoxyphenyl)ethyl]-2-methoxyphenolOther names(R)-(-)-Combretastatin
5-[(2R)-2-hydroxy-2-(3,4,5-trimethoxyphenyl)ethyl]-2-methoxyphenolOther names(R)-(-)-CombretastatinIdentifiers CAS number 82855-09-2 PubChem 9895264 ChemSpider 297705 ChEMBL CHEMBL151766 Jmol-3D images Image 1 - O(c1cc(cc(OC)c1OC)C(O)Cc2ccc(OC)c(O)c2)C
- InChI=1/C18H22O6/c1-21-15-6-5-11(8-14(15)20)7-13(19)12-9-16(22-2)18(24-4)17(10-12)23-3/h5-6,8-10,13,19-20H,7H2,1-4H3
Key: LGZKGOGODCLQHG-UHFFFAOYAS
Properties Molecular formula C18H22O6 Molar mass 334.36 g/mol Exact mass 334.141638 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references The combretastatins are a class of natural stilbenoid natural phenols. A variety of different natural combretastatin molecules are present in the bark of Combretum caffrum, commonly known as South African Bush Willow. Despite having a similar name combretastatin is unrelated to statins, a family of cholesterol lowering drugs.
Contents
Natural combretastatins
Molecules that fall into the combretastatin family generally share 3 common structural features: a trimethoxy "A"-ring, a "B"-ring containing substitutents often at C3' and C4', and an ethene bridge between the two rings which provides necessary structural rigidity.[1] Molecules with C3' amino and hydroxyl substituents are very active, and molecules with C4' hydroxyl or methoxy substituents are also cytotoxic. Of the natural products presently known combretastatin A-4 is the most potent in regards to both tubulin binding ability and cytotoxicity. Combretastatin A-1 is also a potent cytotoxic agent.
Biological function
Members of the combretastatin family possess varying ability to cause vascular disruption in tumors. Combretastatin binds to the β-subunit of tubulin at what is called the colchicine site, referring to the previously discovered vascular disrupting agent colchicine. Inhibition of tubulin polymerization prevents cancer cells from producing microtubules. Microtubules are essential to cytoskeleton production, intercellular movement, cell movement, and formation of the mitotic spindle used in chromosome segregation and cellular division. The anti-cancer activity from this action results from a change in shape in vasculature endothelial cells. Endothelial cells treated with combretastatin rapidly balloon in shape causing a variety of effects which result in necrosis of the tumor core. The tumor edge is supported by normal vasculature and remains, for the most part, unaffected. As a result it is likely that any therapeutic use will involve a combination of drugs or treatment options.
Synthesis
A variety of possible routes to the combretastatin skeleton are possible. One reasonably easy synthesis is as follows:
- 1-Bromomethyl-3,4,5-trimethoxybenzene undergoes an SN2 reaction with triphenylphosphine, which yields a phosphonium salt.
- This compound, through an ylide intermediate, is coupled to a benzaldehyde-derived B-ring possessing the desired substitutents using a Wittig olefination.
- The Wittig reaction produces varying amounts of E and Z isomers depending mainly on solvent polarity, temperature, metal cation coordination effects, and the electronic effect of substituents on either the triphenylphosphine salt or the benzaldehyde. Generally cis-combretastatin possesses significantly improved ability to inhibit tubulin polymerization as well as cytotoxicity.
Clinical studies
Combretastatin A-4, the most potent naturally occurring combretastatin known, its phosphate prodrug (CA-4-P), and other analogs of CA-4 such as ombrabulin are currently being investigated in a number of clinical trials. In July 2007 the pharmaceutical company OXiGENE initiated a 180-patient phase III clinical trial of CA-4-P in combination with carboplatin for the treatment of anaplastic thyroid cancer.[2] There is currently no fully FDA approved treatment for this form of cancer.
References
- ^ Singh, Rohit; Kaur, Harneet (2009). "Advances in Synthetic Approaches for the Preparation of Combretastatin based Anti-Cancer Agents". Synthesis: 2471–2491.
- ^ Study of Combretastatin and Paclitaxel/Carboplatin in the Treatment of Anaplastic Thyroid Cancer
Categories:- Stilbenoids
- Phenol ethers
- Alcohols
Wikimedia Foundation. 2010.