- Treatment of multiple sclerosis
-
Several therapies for multiple sclerosis (MS) exist, although there is no known cure. Multiple sclerosis is a chronic inflammatory demyelinating disease that affects the central nervous system (CNS).
The most common initial course of the disease is the relapsing-remitting subtype, which is characterized by unpredictable attacks (relapses) followed by periods of relative remission with no new signs of disease activity. After some years, many of the people who have this subtype begin to experience neurologic decline without acute relapses. When this happens it is called secondary progressive multiple sclerosis. Other, less common, courses of the disease are the primary progressive (decline from the beginning without attacks) and the progressive-relapsing (steady neurologic decline and superimposed attacks). Different therapies are used for patients experiencing acute attacks, for patients who have the relapsing-remitting subtype, for patients who have the progressive subtypes, for patients without a diagnosis of MS who have a demyelinating event, and for managing the various consequences of MS.
The primary aims of therapy are returning function after an attack, preventing new attacks, and preventing disability. As with any medical treatment, medications used in the management of MS may have several adverse effects, and many possible therapies are still under investigation. At the same time different alternative treatments are pursued by many patients, despite the paucity of supporting, comparable, replicated scientific study.
This article focuses on therapies for standard MS; borderline forms of MS have particular treatments that are excluded.
Contents
Management of acute attacks
During symptomatic attacks, patients may be hospitalized. As of 2007, administration of high doses of intravenous corticosteroids, such as methylprednisolone,[1][2] is the routine therapy for acute relapses. This is administered over a period of three to five days, and has a well-established efficacy in promoting a better recovery from disability.[3][4]
The aim of this kind of treatment is to end the attack sooner and leave fewer lasting deficits in the patient. Although generally effective in the short term for relieving symptoms, corticosteroid treatments do not appear to have a significant impact on long-term recovery: steroids produce a rapid improvement from disability, but this improvement only lasts up to thirty days following a clinical attack and is not evident thirty-six months after the attack. This treatment does not reduce the number of patients who subsequently develop a clinical relapse.[5]
Potential side effects include osteoporosis[6] and impaired memory, the latter being reversible.[7]
Recent studies suggest that steroids administered orally are just as effective at treating MS symptoms as intravenous treatment. However, short-term treatment with high-dose intravenous corticosteroids does not seem to be attended by adverse effects, whereas gastrointestinal symptoms and psychiatric disorders are more common with oral corticosteroids.[8]
Disease-modifying treatments
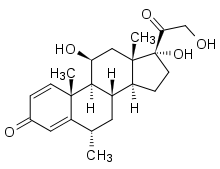
As of 2011, six disease-modifying treatments have been approved by regulatory agencies of different countries, including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMEA) and the Japanese PMDA. The six drugs are two interferons, glatiramer acetate (copaxone), mitoxantrone, natalizumab (tysabri) and Fingolimod (Gilenya). Most of these drugs are approved only for Relapsing-Remitting course.
Clinically isolated syndrome
The earliest clinical presentation of relapsing-remitting MS (RRMS) is the clinically isolated syndrome (CIS), that is, a single attack of a single symptom. During a CIS, there is a subacute attack suggestive of demyelination but the patient does not fulfill the criteria for diagnosis of multiple sclerosis.[9] Several studies have shown that treatment with interferons during an initial attack can decrease the chance that a patient will develop clinical definite MS. These results support the use of interferon after a first clinical demyelinating event and indicate that there may be modest beneficial effects of immediate treatment compared with delayed initiation of treatment.[10][11][12]
Relapsing-remitting MS
The two approved interferons are the interferon beta-1a (with two commercial formulations, with trade names Avonex and Rebif; the first injected weekly, the latter three times a week),[13][14] and the interferon beta-1b (U.S. trade name Betaseron, in Europe and Japan Betaferon),[15] injected every second day.
The other approved drugs are Glatiramer acetate or Copaxone,[16], injected daily[17] , which is a mixture of polypeptides which may protect important myelin proteins by substituting itself as the target of immune system attack. Mitoxantrone is an immunosuppressant also used in cancer chemotherapy. Natalizumab, marketed as Tysabri is a monoclonal antibody [18] and finally Fingolimod (trade name Gilenya) is a sphingosine-1-phosphate receptor modulator[19][20]
All six approved medications differ in their efficacy rate and studies of their long-term effects are still lacking.[21][22][23][24]
The percentage of non-responsive patients to each medication also varies, being around 30% with interferons.[25] Comparisons between immunomodulators (all but mitoxantrone) show that the most effective is natalizumab in terms of relapse rate reduction.[26] Preliminary data points to an effect in disease progression, but studies on the long-term effect are needed.[27] Mitoxantrone is probably the most effective of them all in the short term;[28] however, its use is limited by severe cardiotoxicity,[29] and it is not considered as a long-term therapy. This is the reason why it is mainly used to treat patients who have advanced relapsing-remitting or secondary progressive multiple sclerosis.
Not all the patients are responsive to all these therapies. In particular, a subset of RRMS patients with specially active MS, sometimes called "Rapidly Worsening MS" are normally non-responders to all immunomodulators and are treated with immunosuppressants, in particular, Mitoxantrone.[30][31] To speak about degree of response to treatment, the concept has to be defined first.[32] Several measures have been proposed but none is widely accepted.[33] Nevertheless, the concept is widely used. For example, it is known that 30% of MS patients are non-responsive to Beta interferon.[34] They can be classified in genetic, pharmacological and pathogenetic non-responders.[34]
Even with appropriate use of medication, to varying degrees most patients with relapsing-remitting MS still suffer from some attacks and many suffer subsequent disability.
Secondary progressive MS and progressive relapsing MS
Treatment of advanced forms of MS is more difficult than relapsing-remitting MS. A wide range of medications have been used to try to slow the progression of the disease, with results that have been at best fair.
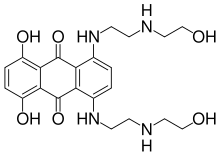
Mitoxantrone has shown positive effects in patients with a secondary progressive and progressive relapsing courses. It is moderately effective in reducing the progression of the disease and the frequency of relapses in patients in short-term follow-up.[35] In 2007 it was the only medication approved in the USA for both secondary progressive and progressive relapsing multiple sclerosis; however, it causes dose-dependent cardiac toxicity which limits its long-term use. It is also not approved in Europe.
Natalizumab or Tysabri has shown efficacy and has been approved for secondary progressive MS with relapses.
Interferon-beta-1b (Betaseron or Betaferon) slowed progression of the disease in one clinical trial for secondary progressive MS, but not in another. However, both studies demonstrated that interferon recipients had fewer relapses and less disease activity, as assessed by magnetic resonance imaging (MRI). Therefore, interferons show promise in treating secondary progressive MS, but more data is needed to support their widespread use.[36]
Primary progressive MS
Treatment of primary progressive multiple sclerosis (PPMS) is problematic as many patients do not respond to any available therapy, and no treatment has been approved specifically for use in this form of the disease.
Several trials have been designed specifically for PPMS, including trials with interferons and mitoxantrone, a phase III trial of glatiramer acetate, and an open-label study of riluzole.[37] Patients with PPMS have also been included in trials of azathioprine,[38] methotrexate,[39] cladribine,[40] intravenous immunoglobulin, cyclophosphamide,[41] and studies of hematopoietic stem cell transplantation. However, no treatment in these trials has been shown to modify the course of the disease.[42]
Side effects of treatments
Both the interferons and glatiramer acetate are available only in injectable forms, and both can cause irritation at the injection site. Also over time, a visible dent at the injection site due to the local destruction of fat tissue, known as lipoatrophy, may develop.
Interferons are produced in the body during illnesses such as influenza in order to help fight the infection.[43] They are responsible for the fever, muscle aches, fatigue, and headache common during influenza infections. Many patients report influenza-like symptoms when using interferon to fight MS. This reaction often lessens over time and can be treated with over-the-counter fever reducers/pain relievers like paracetamol (known in the U.S. as acetaminophen),[44] ibuprofen,[45] and naproxen.[46] Rare, but potentially serious, liver function abnormalities have also been reported with interferons, requiring that all patients treated regularly be monitored with liver function tests to ensure safe use.[47][48][49][50][51][52] Interferon therapy has also been shown to induce the production of anti-IFN neutralizing antibodies (NAb), usually in the second 6 months of treatment, in 3 to 45% of treated patients. However, the clinical consequences of the presence of antibodies are presently unclear: it has not been proved that these antibodies reduce efficacy of treatment. Therefore, any treatment decision should be based only on the clinical response to therapy.[53]
Glatiramer acetate is generally considered to be better tolerated than the interferons, although some patients taking glatiramer experience a post-injection reaction manifested by flushing, chest tightness, heart palpitations, breathlessness, and anxiety, which usually lasts less than thirty minutes.[54]
Mitoxantrone therapy may be associated with immunosuppressive effects and liver damage; however its most dangerous side effect is its dose-related cardiac toxicity. Careful adherence to the administration and monitoring guidelines is therefore essential; this includes obtaining an echocardiogram and a complete blood count before treatment to decide whether the therapy is suitable for the patient or the risks are too great. It is recommended that mitoxantrone be discontinued at the first signs of heart damage, infection or liver dysfunction during therapy.[55]
In the phase III studies for both MS and Crohn's Disease, natalizumab was highly effective and well tolerated; however, three cases of progressive multifocal leukoencephalopathy (PML), a rare progressive demyelinating disease of the brain that typically causes permanent disability or death, were identified in patients; two who had received it in combination with interferons,[56][57] the other a Crohn's Disease patient who had received it in combination with multiple other immuno-suppressants. As a result of a safety evaluation showing that no such cases had occurred in patients treated with natalizumab alone, it was approved as a monotherapy for MS patients.[58] In August 2008, two further cases of PML were reported, one of which had not taken any other immunomodulatory treatment before.[59]
Managing the effects of MS
Disease-modifying treatments only reduce the progression rate of the disease but do not stop it. As multiple sclerosis progresses, the symptoms tend to increase. The disease is associated with a variety of symptoms and functional deficits that result in a range of progressive impairments and handicap. Management of these deficits is therefore very important.
Both drug therapy and neurorehabilitation have shown to ease the burden of some symptoms, even though neither influence disease progression. For other symptoms the efficacy of treatments is still very limited.[60]
Neurorehabilitation
Although there are relatively few studies of rehabilitation in MS,[61][62] its general effectiveness, when conducted by a team of specialists, has been clearly demonstrated in other pathologies such as stroke[63] or head trauma.[64] As for any patient with neurologic deficits, a multidisciplinary approach is key to limiting and overcoming disability; however there are particular difficulties in specifying a ‘core team’ because people with MS may need help from almost any health profession or service at some point.[65] Neurologists are mainly involved in the diagnosis and ongoing management of multiple sclerosis, and any exacerbations. The comprehensive rehabilitation process for patients with multiple sclerosis is generally managed by physiatrists. Allied treatments such as physiotherapy,[66][67] speech and language therapy[68] or occupational therapy[69] can also help to manage some symptoms and maintain quality of life. Treatment of neuropsychiatric symptoms such as emotional distress and clinical depression should involve mental health professionals such as therapists, psychologists, and psychiatrists,[70] while neuropsychologists can help to evaluate and manage cognitive deficits.[71] Multidisciplinary approaches have been shown to be effective in increasing activity levels and participation in multiple sclerosis.[72][73] Due to the paucity of randomized controlled studies, there is limited evidence of the overall efficacy of individual therapy disciplines,[74][74][75] though there is good evidence that specific approaches, such as exercise,[76][77] psychology therapies, particularly cognitive behavioral approaches[78] and energy conservation instruction[79] are effective. More specifically psychological interventions seem useful in the treatment of depression, while evidence on effectiveness for other uses such as the treatment of cognitive impairments or vocational counseling is less strong.[80][81]
Medical treatments for symptoms
Further information: Multiple sclerosis signs and symptomsMultiple sclerosis can cause a variety of symptoms including changes in sensation (hypoesthesia), muscle weakness, abnormal muscle spasms, impaired movement, difficulties with coordination and balance, problems in speech (known as dysarthria) or swallowing (dysphagia), visual problems (nystagmus, optic neuritis, or diplopia), fatigue and acute or chronic pain syndromes, bladder and bowel difficulties, cognitive impairment, or emotional symptoms (mainly depression). The most common clinical measure of disability progression and severity of the symptoms is the Expanded Disability Status Scale or EDSS.[82] At the same time for each symptom there are different treatment options. Treatments should therefore be individualized depending both on the patient and the physician.
- Bladder: pharmacological treatments for bladder problems vary greatly depending on the origin or type of dysfunction; however, some examples of medications used are:[83] alfuzosin for retention,[84] anticholinergics such as trospium and flavoxate for urgency and incontinence,[85][86] or desmopressin for nocturia.[87][88] Non-pharmacological treatments include pelvic floor muscle training, stimulation, biofeedback, pessaries, bladder retraining, and sometimes intermittent catheterization.[89]
- Bowel: people with MS may suffer bowel problems in two ways: reduced gut mobility may follow from immobility and from the drugs used to treat various impairments; and neurological control of defecation may be directly impaired.[65] Pain or problems with defecation can be helped with a diet change, oral laxatives or suppositories and enemas.[90]
- Cognitive and emotional: neuropsychiatric symptomatology is common in the course of the disease. Depression and anxiety appear in up to 80% of patients,[91] and can be treated with a variety of antidepressants;[92] selective serotonin reuptake inhibitors (SSRIs) are the most frequently employed.[93] Other neuropsychiatric symptoms are euphoria and disinhibition. This dyad was called "euphoria sclerotica" by the first authors that described the disease during the 19th century, and affects 10% of patients.[94][95] Anticholinesterase drugs such as donepezil[96]—commonly used in Alzheimer disease—although not approved yet for multiple sclerosis, have shown efficacy in improving cognitive functions.[97][98] Memantine, which is also used in Alzheimer's disease, has been reported to induce reversible neurological impairment that led to stop an ongoing clinical trial.[99] Psychological interventions are also useful in the treatment of cognitive and emotional deficits.[100][101][102][103]
- Dysphagia and dysarthria: dysphagia is a difficulty with eating and swallowing which may cause choking and aspiration of food or liquid into the lungs, while dysarthria is a neurological motor speech disorder characterized by poor control over the subsystems and muscles responsible for speech ("articulation"). A speech and language therapist may give advice on specific swallowing techniques, on adapting food consistencies and dietary intake, on techniques to improve and maintain speech production and clarity, and on alternative communication approaches.[65][68] In the case of advanced dysphagia, food can be supplied by a nasogastric tube, which is a tube that goes through the nose directly to the stomach; or a percutaneous endoscopic gastrostomy (PEG), which is a procedure for placing a tube into the stomach and therefore administering food directly to it. This second system, although more invasive, has better results in the long term than nasogastric intake.[104]
- Fatigue: fatigue is very common and disabling in MS, and at the same time it has a close relationship with depressive symptomatology.[105] When depression is reduced fatigue also tends to improve, so patients should be evaluated for depression before other therapeutic approaches are used.[106] In a similar way, other factors such as disturbed sleep, chronic pain, poor nutrition, or even some medications can contribute to fatigue; medical professionals are therefore encouraged to identify and modify them.[65] A few medications have been studied to treat MS-related fatigue, such as amantadine[107][108] or pemoline (which is a psychostimulant also used for attention-deficit hyperactivity disorder and narcolepsy),[109][110][111] as well as psychological interventions of energy conservation,[112][113] but the effects of all of them are small. Fatigue is therefore a very difficult symptom to manage for which no drugs are recommended.[107]
- Pain: acute pain is mainly due to optic neuritis (with corticosteroids being the best treatment available), as well as trigeminal neuralgia, Lhermitte's sign, or dysesthesias.[114] Subacute pain is usually secondary to the disease and can be a consequence of spending too long in the same position, urinary retention, and infected skin ulcers, amongst others. Treatment will depend on cause. Chronic pain is very common and harder to treat as its most common cause is dysesthesias. Acute pain due to trigeminal neuralgia is usually successfully treated with anticonvulsants such as carbamazepine[115] or phenytoin.[116][117][118] Both Lhermitte's sign and painful dysesthesias usually respond to treatment with carbamazepine, clonazepam,[119] or amitriptyline.[120][121] Sativex is approved for treatment of pain in MS in different countries, but due to its derivation from cannabis, it is currently not available in others, such as the USA.[122] This medication is also being investigated for the management of other MS symptoms, such as spasticity,[123] and has shown long-term safety and efficacy.[124]
- Spasticity: spasticity is characterized by increased stiffness and slowness in limb movement, the development of certain postures, an association with weakness of voluntary muscle power, and with involuntary and sometimes painful spasms of limbs.[65] A physiotherapist can help to reduce spasticity and avoid the development of contractures with techniques such as passive stretching.[125] There is evidence, albeit limited, of the clinical effectiveness of baclofen,[126] dantrolene,[127] diazepam,[128] and tizanidine.[129][130][131] In the most complicated cases intrathecal injections of baclofen can be used.[132] There are also palliative measures like castings, splints or customized seatings.[65]
- Vision: different drugs as well as optic compensatory systems and prisms can be used to improve the symptoms of nystagmus or diplopia (double vision).[133][134][135] Surgery can also be used in some cases.[136]
- Walking capacity: dalfampridine (ampyra) improves walking ability and is approved by the FDA.[137]
Unfortunately, other symptoms, such as ataxia, tremor or sensory losses, do not have proven treatments.[65]
Therapies under investigation
Main article: Therapies under investigation for multiple sclerosisScientists continue their extensive efforts to create new and better therapies for MS. There are a number of treatments under investigation that may curtail attacks or improve function. Some of these treatments involve the combination of drugs that are already in use for multiple sclerosis, such as the joint administration of mitoxantrone and glatiramer acetate (Copaxone).[138]
However most treatments already in clinical trials involve drugs that are used in other diseases. These are the cases of alemtuzumab (Campath),[139] daclizumab (Zenapax),[140] inosine,[141] or BG00012.[142] Alemtuzumab performed better than interferon beta-1a in relapsing-remitting MS reducing disability, imaging abnormalities and frequence of relapses, at the cost of increased autoimmunity problems. These included three cases of thrombocytopenic purpura which led to the suspension of the therapy.[143] Other drugs in clinical trials have been designed specifically for MS, such as fingolimod,[144] laquinimod,[145] or Neurovax.[146]
In humans, BCG vaccine, the common, live, attenuated vaccine against tuberculosis, has substantially reduced recurrence of symptoms in multiple sclerosis patients.[147] The frequency of new enhancing lesions as detected by Gd-enhanced MRI was reduced by more than half in 12 patients, comparing the six-month run-in phase to the six-month post BCG phase of the experiment. Persistence at subsequent MR scan was reduced from 18 to 1 lesion, and evolution to black holes was reduced from 28 to 6 lesions.[148] The conventional explanation of such protection is that parasites (including bacteria) modulate the sensitivity of the immune system. BCG appears safe as a treatment for multiple sclerosis.[147][149]
Many anecdotes are found on the Internet about the effectiveness of low dose naltrexone for MS, but no published scientific studies or case reports address its effectiveness.[150] Finally, there are also many early-stage investigations that in the future may emerge as new treatments. Examples of these are the studies trying to understand the influence of Chlamydophila pneumoniae or vitamin D in the origin of the disease,[151][152] or preliminary investigations on the use of helminthic therapy,[153] or angioplasty and venous stents based on the theory that an incorrect blood drainage system weakens the blood-brain barrier.[154]
Alternative treatments
A recent study found that over 60% of MS patients use complementary and alternative medicine, possibly because conventional treatments lack effectiveness. Except for vitamin D, evidence is lacking for these treatments and there are no clear guidelines for their use.[155]
The effect of diet on MS is unclear, with studies unable to show whether diet contributes to MS or can alter its course. Ecologic studies have found a link between high consumptions of polyunsaturated fats and low MS prevalence. The most promising evidence comes from daily supplementation with vitamin D. Evidence is lacking for several other widely promoted non-herbal supplements, including vitamin B12, alpha-lipoic acid, luteolin, and evening primrose oil.[155] Many diets have been proposed for treating the symptoms of the disease. Patients have reported a decrease in symptoms after long-term application of changes in diet; however, no controlled trials have been able to prove their efficacy.[156] Even if these diets are genuinely beneficial for people with MS, it is not known whether this is due to any special traits for MS, as opposed to their known benefits for whole body health.[155] Two examples of such diets are the Swank Multiple Sclerosis Diet[157][158] and The Best Bet Diet.
Herbal medicine is another source of alternative treatments. Many patients use medical marijuana to help alleviate symptoms; however, the results of experimental studies are scarce. At least one subgroup experiencing greater disability appears to have derived some symptomatic benefit.[159][160] Derivatives from the herb common rue (Ruta graveolens); which contain compounds that block Kv1.3 channels in T cells; have also been suggested to ameliorate MS symptoms.[161][162] Kv1.3 channel-blockers are in development for the treatment of the disease.[163][164]
Bee venom therapy has been promoted as a possible treatment for MS, as it is thought to be an anti-inflammatory and inflammation is a component of MS. Research results have been variable and its benefit has not been demonstrated. Its greatest safety issue is the risk of anaphylactic shock, as about 15% of the population is allergic to bee stings.[155]
Hyperbaric oxygenation has been the subject of several small studies with heterogeneous results which, overall, do not support its use.[165]
Relaxation disciplines such as yoga, and general exercise, seem to mitigate fatigue and improve quality of life.[166] Some studies also show benefits on physical variables, such as balance and strength or cardiovascular and respiratory function, but more investigation is needed as the studies are usually of low quality.[167]
Further reading
Clinical guidelines: clinical guidelines are documents with the aim of guiding decisions and criteria in specific areas of healthcare, as defined by an authoritative examination of current evidence (evidence-based medicine).
- The Royal College of Physicians (2004). Multiple Sclerosis. National clinical guideline for diagnosis and management in primary and secondary care. Salisbury, Wiltshire: Sarum ColourView Group. ISBN 1 86016 182 0.Free full text (2004-08-13). Retrieved on 2007-10-01.
- Multiple sclerosis. Understanding NICE guidance. Information for people with multiple sclerosis, their families and carers, and the public. London: National Institute of Clinical Excellence. 2003. ISBN 1-84257-445-0. Free full text (2003-11-26). Retrieved on 2007-10-25.
Notes and references
- ^ Methylprednisolone Oral. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-01.[dead link][dead link] Archived September 1, 2007 at the Wayback Machine
- ^ Methylprednisolone Sodium Succinate Injection. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-01.[dead link][dead link] Archived September 14, 2007 at the Wayback Machine
- ^ Sellebjerg F, Barnes D, Filippini G, et al. (2005). "EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses". Eur. J. Neurol. 12 (12): 939–46. doi:10.1111/j.1468-1331.2005.01352.x. PMID 16324087.
- ^ Goodin DS, Frohman EM, Garmany GP, et al. (2002). "Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines". Neurology 58 (2): 169–78. PMID 11805241.
- ^ Brusaferri F, Candelise L (2000). "Steroids for multiple sclerosis and optic neuritis: a meta-analysis of randomized controlled clinical trials". J. Neurol. 247 (6): 435–42. doi:10.1007/s004150070172. PMID 10929272.
- ^ Dovio A, Perazzolo L, Osella G, et al. (2004). "Immediate fall of bone formation and transient increase of bone resorption in the course of high-dose, short-term glucocorticoid therapy in young patients with multiple sclerosis". J. Clin. Endocrinol. Metab. 89 (10): 4923–8. doi:10.1210/jc.2004-0164. PMID 15472186.
- ^ Uttner I, Müller S, Zinser C, et al. (2005). "Reversible impaired memory induced by pulsed methylprednisolone in patients with MS". Neurology 64 (11): 1971–3. doi:10.1212/01.WNL.0000163804.94163.91. PMID 15955958.
- ^ Filippini G, Brusaferri F, Sibley WA, et al. (2000). Filippini, Graziella. ed. "Corticosteroids or ACTH for acute exacerbations in multiple sclerosis". Cochrane database of systematic reviews (Online) (4): CD001331. doi:10.1002/14651858.CD001331. PMID 11034713.
- ^ Miller D, Barkhof F, Montalban X, Thompson A, Filippi M (2005). "Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis". Lancet neurology 4 (5): 281–8. doi:10.1016/S1474-4422(05)70071-5. PMID 15847841.
- ^ Jacobs LD, Beck RW, Simon JH, et al. (2000). "Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group". N Engl J Med 343 (13): 898–904. doi:10.1056/NEJM200009283431301. PMID 11006365.
- ^ Comi G, Filippi M, Barkhof F, et al. (2001). "Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study". Lancet 357 (9268): 1576–82. doi:10.1016/S0140-6736(00)04725-5. PMID 11377645.
- ^ Kappos L, Freedman MS, Polman CH, et al. (2007). "Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study". Lancet 370 (9585): 389–97. doi:10.1016/S0140-6736(07)61194-5. PMID 17679016.
- ^ Interferon beta-1a Intramuscular Injection. US National Library of Medicine (Medline) (2006-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 6, 2007 at the Wayback Machine
- ^ Interferon beta-1a Subcutaneous Injection. US National Library of Medicine (Medline) (2004-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived August 27, 2007 at the Wayback Machine
- ^ Interferon Beta-1b Injection. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-09-02[dead link][dead link]
- ^ Glatiramer injection. US National Library of Medicine (Medline) (2003-07-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 11, 2007 at the Wayback Machine
- ^ Ziemssen T, Schrempf W (2007). "Glatiramer acetate: mechanisms of action in multiple sclerosis". Int. Rev. Neurobiol.. International Review of Neurobiology 79: 537–70. doi:10.1016/S0074-7742(07)79024-4. ISBN 9780123737366. PMID 17531858.
- ^ Natalizumab Injection. US National Library of Medicine (Medline) (2006-10-01). Retrieved on 2007-09-02.[dead link] Archived September 11, 2007 at the Wayback Machine
- ^ Cohen J, et al., NEJM 2010
- ^ Information on the phase III trial for fingolimod
- ^ Ruggieri M, Avolio C, Livrea P, Trojano M (2007). "Glatiramer acetate in multiple sclerosis: a review". CNS Drug Rev 13 (2): 178–91. doi:10.1111/j.1527-3458.2007.00010.x. PMID 17627671.
- ^ Munari L, Lovati R, Boiko A (2004). Munari, Luca M.. ed. "Therapy with glatiramer acetate for multiple sclerosis". Cochrane Database Syst Rev (1): CD004678. doi:10.1002/14651858.CD004678. PMID 14974077.
- ^ Rice GP, Incorvaia B, Munari L, et al. (2001). Filippini, Graziella. ed. "Interferon in relapsing-remitting multiple sclerosis". Cochrane Database Syst Rev (4): CD002002. doi:10.1002/14651858.CD002002. PMID 11687131.
- ^ Martinelli Boneschi F, Rovaris M, Capra R, Comi G (2005). Martinelli Boneschi, Filippo. ed. "Mitoxantrone for multiple sclerosis". Cochrane Database Syst Rev (4): CD002127. doi:10.1002/14651858.CD002127.pub2. PMID 16235298.
- ^ Fernández O, Fernández V, Mayorga C, et al. (2005). "HLA class II and response to interferon-beta in multiple sclerosis". Acta Neurol. Scand. 112 (6): 391–4. doi:10.1111/j.1600-0404.2005.00415.x. PMID 16281922.
- ^ Johnson KP (2007). "Control of multiple sclerosis relapses with immunomodulating agents". J. Neurol. Sci. 256 Suppl 1: S23–8. doi:10.1016/j.jns.2007.01.060. PMID 17350652.
- ^ Natalizumab reduces MS severity
- ^ Gonsette RE (2007). "Compared benefit of approved and experimental immunosuppressive therapeutic approaches in multiple sclerosis". Expert opinion on pharmacotherapy 8 (8): 1103–16. doi:10.1517/14656566.8.8.1103. PMID 17516874.
- ^ Murray TJ (2006). "The cardiac effects of mitoxantrone: do the benefits in multiple sclerosis outweigh the risks?". Expert opinion on drug safety 5 (2): 265–74. doi:10.1517/14740338.5.2.265. PMID 16503747.
- ^ Buttinelli C, Clemenzi A, Borriello G, Denaro F, Pozzilli C, Fieschi C. (2007). "Mitoxantrone treatment in multiple sclerosis: a 5-year clinical and MRI follow-up". European Journal of Neurology 14 (11): 1281–7. doi:10.1111/j.1468-1331.2007.01969.x. PMID 17956449.
- ^ Boster A, Edan G, Frohman E, Javed A, Stuve O, Tselis A, Weiner H, Weinstock-Guttman B, Khan O (2008). "Intense immunosuppression in patients with rapidly worsening multiple sclerosis: treatment guidelines for the clinician". Lancet neurology 7 (2): 173–83. doi:10.1016/S1474-4422(08)70020-6. PMID 18207115.
- ^ Comi G (September 2008). "Definition of responder: introduction". Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 29 Suppl 2: S209–10. doi:10.1007/s10072-008-0938-x. PMID 18690493.
- ^ Furlan R (September 2008). "Definition of non-responders: biological markers". Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 29 Suppl 2: S214–5. doi:10.1007/s10072-008-0940-3. PMID 18690495.
- ^ a b Bertolotto A, Gilli F (September 2008). "Interferon-beta responders and non-responders. A biological approach". Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 29 Suppl 2: S216–7. doi:10.1007/s10072-008-0941-2. PMID 18690496.
- ^ Martinelli Boneschi F, Rovaris M, Capra R, Comi G (2005). Martinelli Boneschi, Filippo. ed. "Mitoxantrone for multiple sclerosis". Cochrane database of systematic reviews (Online) (4): CD002127. doi:10.1002/14651858.CD002127.pub2. PMID 16235298.
- ^ McCormack PL, Scott LJ (2004). "Interferon-beta-1b: a review of its use in relapsing-remitting and secondary progressive multiple sclerosis". CNS drugs 18 (8): 521–46. PMID 15182221.
- ^ Riluzole. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived August 19, 2007 at the Wayback Machine
- ^ Azathioprine. US National Library of Medicine (Medline) (2004-04-01). Retrieved on 2007-09-02.[dead link] Archived September 1, 2007 at the Wayback Machine
- ^ Methotrexate. US National Library of Medicine (Medline) (2006-10-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 5, 2007 at the Wayback Machine
- ^ Cladribine. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived August 16, 2007 at the Wayback Machine
- ^ Cyclophosphamide. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 5, 2007 at the Wayback Machine
- ^ Leary SM, Thompson AJ (2005). "Primary progressive multiple sclerosis: current and future treatment options". CNS drugs 19 (5): 369–76. doi:10.2165/00023210-200519050-00001. PMID 15907149.
- ^ Sládková T, Kostolanský F (2006). "The role of cytokines in the immune response to influenza A virus infection". Acta Virol. 50 (3): 151–62. PMID 17131933.
- ^ Acetaminophen. US National Library of Medicine (Medline) (2007-07-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 25, 2007 at the Wayback Machine
- ^ Ibuprofen. US National Library of Medicine (Medline) (2007-03-01). Retrieved on 2007-09-02.[dead link] Archived August 29, 2007 at the Wayback Machine
- ^ Naproxen. US National Library of Medicine (Medline) (2006-01-01). Retrieved on 2007-09-02.[dead link][dead link] Archived August 4, 2007 at the Wayback Machine
- ^ Betaseron [package insert]. Montville, NJ: Berlex Inc; 2003
- ^ Betaseron FDA label - http://www.fda.gov/cder/foi/label/2003/103471s5032lbl.pdf[dead link][dead link] Archived November 20, 2006 at the Wayback Machine
- ^ Rebif [package insert]. Rockland, MA: Serono Inc; 2005.
- ^ Rebif FDA label -http://www.fda.gov/cder/foi/label/2003/ifnbser050203LB.pdf[dead link][dead link] Archived August 16, 2005 at the Wayback Machine
- ^ Avonex [package insert]. Cambridge, MA: Biogen Inc; 2003
- ^ Avonex FDA label - http://www.fda.gov/cder/foi/label/2003/ifnbbio052803LB.pdf[dead link][dead link] Archived May 16, 2005 at the Wayback Machine
- ^ Durelli L, Ricci A (2004). "Anti-interferon antibodies in multiple sclerosis. Molecular basis and their impact on clinical efficacy". Front. Biosci. 9: 2192–204. doi:10.2741/1329. PMID 15353281.
- ^ Munari L, Lovati R, Boiko A (2004). Munari, Luca M.. ed. "Therapy with glatiramer acetate for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD004678. doi:10.1002/14651858.CD004678. PMID 14974077.
- ^ Fox EJ (2006). "Management of worsening multiple sclerosis with mitoxantrone: a review". Clinical therapeutics 28 (4): 461–74. doi:10.1016/j.clinthera.2006.04.013. PMID 16750460.
- ^ Kleinschmidt-DeMasters BK, Tyler KL (2005). "Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis". N Engl J Med 353 (4): 369–74. doi:10.1056/NEJMoa051782. PMID 15947079. Free full text with registration
- ^ Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D (2005). "Progressive multifocal leukoencephalopathy in a patient treated with natalizumab". N Engl J Med 353 (4): 375–81. doi:10.1056/NEJMoa051847. PMID 15947078. Free full text with registration
- ^ Kappos L, Bates D, Hartung HP, et al. (2007). "Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring". Lancet neurology 6 (5): 431–41. doi:10.1016/S1474-4422(07)70078-9. PMID 17434098.
- ^ Goldstein, Jacob (2008-08-01). "Brain Infections Return for Multiple Sclerosis Drug Tysabri". The Wall Street Journal. http://blogs.wsj.com/health/2008/08/01/brain-infections-return-for-multiple-sclerosis-drug-tysabri/?mod=googlenews_wsj. Retrieved 2008-08-01.
- ^ Kesselring J, Beer S (2005). "Symptomatic therapy and neurorehabilitation in multiple sclerosis". Lancet neurology 4 (10): 643–52. doi:10.1016/S1474-4422(05)70193-9. PMID 16168933.
- ^ Di Fabio RP, Soderberg J, Choi T, Hansen CR, Schapiro RT (1998). "Extended outpatient rehabilitation: its influence on symptom frequency, fatigue, and functional status for persons with progressive multiple sclerosis". Archives of physical medicine and rehabilitation 79 (2): 141–6. doi:10.1016/S0003-9993(98)90290-8. PMID 9473994.
- ^ Solari A, Filippini G, Gasco P, et al. (1999). "Physical rehabilitation has a positive effect on disability in multiple sclerosis patients". Neurology 52 (1): 57–62. PMID 9921849.
- ^ Langhorne, Peter (2000). Langhorne, Peter. ed. "Organised inpatient (stroke unit) care for stroke. Stroke Unit Trialists' Collaboration". Cochrane database of systematic reviews (Online) (2): CD000197. doi:10.1002/14651858.CD000197. PMID 10796318.
- ^ Turner-Stokes L, Disler PB, Nair A, Wade DT (2005). Turner-Stokes, Lynne. ed. "Multi-disciplinary rehabilitation for acquired brain injury in adults of working age". Cochrane database of systematic reviews (Online) (3): CD004170. doi:10.1002/14651858.CD004170.pub2. PMID 16034923.
- ^ a b c d e f g The Royal College of Physicians (2004). Multiple Sclerosis. National clinical guideline for diagnosis and management in primary and secondary care. Salisbury, Wiltshire: Sarum ColourView Group. ISBN 1 86016 182 0.Free full text (2004-08-13). Retrieved on 2007-10-01.
- ^ Heesen C, Romberg A, Gold S, Schulz KH (2006). "Physical exercise in multiple sclerosis: supportive care or a putative disease-modifying treatment". Expert review of neurotherapeutics 6 (3): 347–55. doi:10.1586/14737175.6.3.347. PMID 16533139.
- ^ Rietberg MB, Brooks D, Uitdehaag BM, Kwakkel G (2005). Kwakkel, Gert. ed. "Exercise therapy for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD003980. doi:10.1002/14651858.CD003980.pub2. PMID 15674920.
- ^ a b Merson RM, Rolnick MI (1998). "Speech-language pathology and dysphagia in multiple sclerosis". Physical medicine and rehabilitation clinics of North America 9 (3): 631–41. PMID 9894114.
- ^ Baker NA, Tickle-Degnen L (2001). "The effectiveness of physical, psychological, and functional interventions in treating clients with multiple sclerosis: a meta-analysis". The American Journal of Occupational Therapy 55 (3): 324–31. doi:10.5014/ajot.55.3.324. PMID 11723974.
- ^ Ghaffar O, Feinstein A (2007). "The neuropsychiatry of multiple sclerosis: a review of recent developments". Curr Opin Psychiatry 20 (3): 278–85. doi:10.1097/YCO.0b013e3280eb10d7. PMID 17415083.
- ^ Benedict RH, Bobholz JH (2007). "Multiple sclerosis". Seminars in neurology 27 (1): 78–85. doi:10.1055/s-2006-956758. PMID 17226744.
- ^ Khan F, Turner-Stokes L, Ng L, Kilpatrick T (2008). "Multidisciplinary rehabilitation for adults with multiple sclerosis". J. Neurol. Neurosurg. Psychiatr. 79 (2): 114. doi:10.1136/jnnp.2007.127563. PMID 18202203.
- ^ Khan F, Turner-Stokes L, Ng L, Kilpatrick T (2007). Khan, Fary. ed. "Multidisciplinary rehabilitation for adults with multiple sclerosis". Cochrane Database of Systematic Reviews (2): CD006036. doi:10.1002/14651858.CD006036.pub2. PMID 17443610.
- ^ a b Steultjens EM, Dekker J, Bouter LM, Leemrijse CJ, van den Ende CH (2005). "Evidence of the efficacy of occupational therapy in different conditions: an overview of systematic reviews". Clinical rehabilitation 19 (3): 247–54. doi:10.1191/0269215505cr870oa. PMID 15859525.
- ^ Steultjens EM, Dekker J, Bouter LM, Cardol M, Van de Nes JC, Van den Ende CH (2003). Steultjens, Esther EMJ. ed. "Occupational therapy for multiple sclerosis". Cochrane database of systematic reviews (Online) (3): CD003608. doi:10.1002/14651858.CD003608. PMID 12917976.
- ^ Gallien P, Nicolas B, Robineau S, Pétrilli S, Houedakor J, Durufle A (2007). "Physical training and multiple sclerosis". Ann Readapt Med Phys 50 (6): 373–6, 369–72. doi:10.1016/j.annrmp.2007.04.004. PMID 17482708.
- ^ Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G (2005). Kwakkel, Gert. ed. "Exercise therapy for multiple sclerosis". Cochrane Database of Systematic Reviews (1): CD003980. doi:10.1002/14651858.CD003980.pub2. PMID 15674920.
- ^ Thomas PW, Thomas S, Hillier C, Galvin K, Baker R (2006). Thomas, Peter W. ed. "Psychological interventions for multiple sclerosis". Cochrane Database of Systematic Reviews (1): CD004431. doi:10.1002/14651858.CD004431.pub2. PMID 16437487.
- ^ Mathiowetz V, Matuska KM, Murphy ME (2001). "Efficacy of an energy conservation course for persons with multiple sclerosis". Arch Phys Med Rehabil 82 (4): 449–56. doi:10.1053/apmr.2001.22192. PMID 11295003.
- ^ Khan F, Ng L, Turner-Stokes L (2009). Khan, Fary. ed. "Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis". Cochrane Database Syst Rev (1): CD007256. doi:10.1002/14651858.CD007256.pub2. PMID 19160331.
- ^ Thomas PW, Thomas S, Hillier C, Galvin K, Baker R (2006). Thomas, Peter W. ed. "Psychological interventions for multiple sclerosis". Cochrane Database Syst Rev (1): CD004431. doi:10.1002/14651858.CD004431.pub2. PMID 16437487.
- ^ Kurtzke JF (1983). "Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS)". Neurology 33 (11): 1444–52. PMID 6685237.
- ^ Ayuso-Peralta L, de Andrés C (2002). "[Symptomatic treatment of multiple sclerosis]" (in Spanish; Castilian). Revista de neurologia 35 (12): 1141–53. PMID 12497297.
- ^ Alfuzosin. US National Library of Medicine (Medline) (2007-03-01). Retrieved on 2007-09-02.[dead link][dead link] Archived June 10, 2007 at the Wayback Machine
- ^ Trospium. US National Library of Medicine (Medline) (2005-01-01). Retrieved on 2007-09-02.[dead link][dead link] Archived October 19, 2007 at the Wayback Machine
- ^ Flavoxate. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 8, 2007 at the Wayback Machine
- ^ Bosma R, Wynia K, Havlíková E, De Keyser J, Middel B (2005). "Efficacy of desmopressin in patients with multiple sclerosis suffering from bladder dysfunction: a meta-analysis". Acta Neurol. Scand. 112 (1): 1–5. doi:10.1111/j.1600-0404.2005.00431.x. PMID 15932348.
- ^ Desmopressin. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 6, 2007 at the Wayback Machine
- ^ Frances M Diro (2006-10-11). Urological Management in Neurological Disease. eMedicine. WebMD. Retrieved on 2007-09-02.
- ^ DasGupta R, Fowler CJ (2003). "Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies". Drugs 63 (2): 153–66. doi:10.2165/00003495-200363020-00003. PMID 12515563.
- ^ Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C (1999). "Neuropsychiatric manifestations of multiple sclerosis". The Journal of neuropsychiatry and clinical neurosciences 11 (1): 51–7. PMID 9990556.
- ^ Gill D, Hatcher S (2007). Gill, David. ed. "WITHDRAWN: Antidepressants for depression in medical illness". Cochrane database of systematic reviews (Online) (3): CD001312. doi:10.1002/14651858.CD001312.pub2. PMID 17636666.
- ^ Selective serotonin reuptake inhibitors. Mayo Clinic (2006-12-08). Retrieved on 2007-09-02.
- ^ Finger S (1998). "A happy state of mind: a history of mild elation, denial of disability, optimism, and laughing in multiple sclerosis". Arch. Neurol. 55 (2): 241–50. doi:10.1001/archneur.55.2.241. PMID 9482369.
- ^ Fishman I, Benedict RH, Bakshi R, Priore R, Weinstock-Guttman B (2004). "Construct validity and frequency of euphoria sclerotica in multiple sclerosis". The Journal of neuropsychiatry and clinical neurosciences 16 (3): 350–6. doi:10.1176/appi.neuropsych.16.3.350. PMID 15377743.
- ^ Donepezil. US National Library of Medicine (Medline) (2007-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 2, 2007 at the Wayback Machine
- ^ Christodoulou C, Melville P, Scherl W, Macallister W, Elkins L, Krupp L (2006). "Effects of donepezil on memory and cognition in multiple sclerosis". J Neurol Sci 245 (1–2): 127–36. doi:10.1016/j.jns.2005.08.021. PMID 16626752.
- ^ Porcel J, Montalban X (2006). "Anticholinesterasics in the treatment of cognitive impairment in multiple sclerosis". J Neurol Sci 245 (1–2): 177–81. doi:10.1016/j.jns.2005.07.021. PMID 16674980.
- ^ Villoslada P, Arrondo G, Sepulcre J, Alegre M, Artieda J (December 2008). "Memantine induces reversible neurologic impairment in patients with MS". Neurology 72 (19): 1630–3. doi:10.1212/01.wnl.0000342388.73185.80. PMID 19092106.
- ^ Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L (2001). "Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis". Journal of consulting and clinical psychology 69 (6): 942–9. doi:10.1037/0022-006X.69.6.942. PMID 11777121.
- ^ Benedict RH, Shapiro A, Priore R, Miller C, Munschauer F, Jacobs L (2000). "Neuropsychological counseling improves social behavior in cognitively impaired multiple sclerosis patients". Mult Scler 6 (6): 391–6. PMID 11212135.
- ^ Thomas PW, Thomas S, Hillier C, Galvin K, Baker R (2006). Thomas, Peter W. ed. "Psychological interventions for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD004431. doi:10.1002/14651858.CD004431.pub2. PMID 16437487.
- ^ Amato MP, Zipoli V (2003). "Clinical management of cognitive impairment in multiple sclerosis: a review of current evidence". International MS journal / MS Forum 10 (3): 72–83. PMID 14561373.
- ^ Bath PM, Bath FJ, Smithard DG (2000). Bath, Philip MW. ed. "Interventions for dysphagia in acute stroke". Cochrane database of systematic reviews (Online) (2): CD000323. doi:10.1002/14651858.CD000323. PMID 10796343.
- ^ Bakshi R (2003). "Fatigue associated with multiple sclerosis: diagnosis, impact and management". Mult. Scler. 9 (3): 219–27. doi:10.1191/1352458503ms904oa. PMID 12814166.
- ^ Mohr DC, Hart SL, Goldberg A (2003). "Effects of treatment for depression on fatigue in multiple sclerosis". Psychosomatic medicine 65 (4): 542–7. doi:10.1097/01.PSY.0000074757.11682.96. PMID 12883103.
- ^ a b Pucci E, Branãs P, D'Amico R, Giuliani G, Solari A, Taus C (2007). Pucci, Eugenio. ed. "Amantadine for fatigue in multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD002818. doi:10.1002/14651858.CD002818.pub2. PMID 17253480.
- ^ Amantadine. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-07.[dead link][dead link] Archived October 7, 2007 at the Wayback Machine
- ^ Weinshenker BG, Penman M, Bass B, Ebers GC, Rice GP (1992). "A double-blind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis". Neurology 42 (8): 1468–71. PMID 1641137.
- ^ Pemoline. US National Library of Medicine (Medline) (2006-01-01). Retrieved on 2007-10-07.[dead link] Archived October 12, 2007 at the Wayback Machine
- ^ Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C (2000). "Treatments for fatigue in multiple sclerosis: a rapid and systematic review". Health Technol Assess 4 (27): 1–61. PMID 11074395. http://www.hta.ac.uk/execsumm/summ427.htm.
- ^ Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P (2005). "Randomized controlled trial of an energy conservation course for persons with multiple sclerosis". Mult Scler 11 (5): 592–601. doi:10.1191/1352458505ms1198oa. PMID 16193899.
- ^ Matuska K, Mathiowetz V, Finlayson M (2007). "Use and perceived effectiveness of energy conservation strategies for managing multiple sclerosis fatigue". The American Journal of Occupational Therapy 61 (1): 62–9. doi:10.5014/ajot.61.1.62. PMID 17302106.
- ^ Kerns RD, Kassirer M, Otis J (2002). "Pain in multiple sclerosis: a biopsychosocial perspective". Journal of rehabilitation research and development 39 (2): 225–32. PMID 12051466.
- ^ Carbamazepine. US National Library of Medicine (Medline) (2007-05-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 5, 2007 at the Wayback Machine
- ^ Phenytoin. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.[dead link] Archived September 12, 2007 at the Wayback Machine
- ^ Brisman R (1987). "Trigeminal neuralgia and multiple sclerosis". Arch. Neurol. 44 (4): 379–81. PMID 3493757.
- ^ Bayer DB, Stenger TG (1979). "Trigeminal neuralgia: an overview". Oral Surg. Oral Med. Oral Pathol. 48 (5): 393–9. doi:10.1016/0030-4220(79)90064-1. PMID 226915.
- ^ Clonazepam. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-09-02.[dead link][dead link] Archived August 27, 2007 at the Wayback Machine
- ^ Amitriptyline. US National Library of Medicine (Medline) (2007-08-01). Retrieved on 2007-09-02.[dead link][dead link] Archived September 2, 2007 at the Wayback Machine
- ^ Moulin DE, Foley KM, Ebers GC (1988). "Pain syndromes in multiple sclerosis". Neurology 38 (12): 1830–4. PMID 2973568.
- ^ Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR (2007). "Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain". Current medical research and opinion 23 (1): 17–24. doi:10.1185/030079906X158066. PMID 17257464.
- ^ Perras C (2005). "Sativex for the management of multiple sclerosis symptoms". Issues in emerging health technologies (72): 1–4. PMID 16317825.
- ^ Wade DT, Makela PM, House H, Bateman C, Robson P (2006). "Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis". Mult. Scler. 12 (5): 639–45. doi:10.1177/1352458505070618. PMID 17086911.
- ^ Cardini RG, Crippa AC, Cattaneo D (2000). "Update on multiple sclerosis rehabilitation". J. Neurovirol. 6 Suppl 2: S179–85. PMID 10871810.
- ^ Baclofen oral. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-17.[dead link][dead link] Archived October 16, 2007 at the Wayback Machine
- ^ Dantrolene oral. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-17.[dead link][dead link] Archived October 18, 2007 at the Wayback Machine
- ^ Diazepam. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-10-17.[dead link][dead link] Archived October 11, 2007 at the Wayback Machine
- ^ Tizanidine. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-10-17.[dead link][dead link] Archived October 21, 2007 at the Wayback Machine
- ^ Beard S, Hunn A, Wight J (2003). "Treatments for spasticity and pain in multiple sclerosis: a systematic review". Health technology assessment (Winchester, England) 7 (40): iii, ix–x, 1–111. PMID 14636486.
- ^ Paisley S, Beard S, Hunn A, Wight J (2002). "Clinical effectiveness of oral treatments for spasticity in multiple sclerosis: a systematic review". Mult. Scler. 8 (4): 319–29. doi:10.1191/1352458502ms795rr. PMID 12166503.
- ^ Becker WJ, Harris CJ, Long ML, Ablett DP, Klein GM, DeForge DA (1995). "Long-term intrathecal baclofen therapy in patients with intractable spasticity". The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques 22 (3): 208–17. PMID 8529173.
- ^ Leigh RJ, Averbuch-Heller L, Tomsak RL, Remler BF, Yaniglos SS, Dell'Osso LF (1994). "Treatment of abnormal eye movements that impair vision: strategies based on current concepts of physiology and pharmacology". Ann. Neurol. 36 (2): 129–41. doi:10.1002/ana.410360204. PMID 8053648.
- ^ Starck M, Albrecht H, Pöllmann W, Straube A, Dieterich M (1997). "Drug therapy for acquired pendular nystagmus in multiple sclerosis". J. Neurol. 244 (1): 9–16. doi:10.1007/PL00007728. PMID 9007739.
- ^ Menon GJ, Thaller VT (2002). "Therapeutic external ophthalmoplegia with bilateral retrobulbar botulinum toxin- an effective treatment for acquired nystagmus with oscillopsia". Eye (London, England) 16 (6): 804–6. doi:10.1038/sj.eye.6700167. PMID 12439689.
- ^ Jain S, Proudlock F, Constantinescu CS, Gottlob I (2002). "Combined pharmacologic and surgical approach to acquired nystagmus due to multiple sclerosis". Am. J. Ophthalmol. 134 (5): 780–2. doi:10.1016/S0002-9394(02)01629-X. PMID 12429265.
- ^ FDA Approves Ampyra to Improve Walking in Adults with Multiple Sclerosis
- ^ United Kingdom early Mitoxantrone Copaxone trial. Onyx Healthcare (2006-01-01). Retrieved on 2007-09-02.
- ^ Genzyme and Bayer HealthCare Announce Detailed Interim Two-Year Alemtuzumab in Multiple Sclerosis Data Presented at AAN. Genzyme (2007-02-01). Retrieved on 2007-09-02.
- ^ Daclizumab. PDL Biopharma (2006-01-01). Retrieved on 2007-09-02.[dead link] Archived September 15, 2007 at the Wayback Machine
- ^ Treatment of Multiple Sclerosis Using Over the Counter Inosine. ClinicalTrials.gov (2006-03-16). Retrieved on 2007-09-02.
- ^ Efficacy and Safety of BG00012 in Relapsing-Remitting Multiple Sclerosis. ClinicalTrials.gov (2007-09-01). Retrieved on 2007-11-12.
- ^ The CAMMS223 Trial Investigators (2008). "Alemtuzumab vs. Interferon Beta-1a in Early Multiple Sclerosis". N Engl J Med 359 (17): 1786–1801. doi:10.1056/NEJMoa0802670. PMID 18946064.
- ^ Efficacy and Safety of Fingolimod in Patients With Relapsing-Remitting Multiple Sclerosis. ClinicalTrials.gov (2006-02-09). Retrieved on 2007-09-02.
- ^ Polman C, Barkhof F, Sandberg-Wollheim M, Linde A, Nordle O, Nederman T (2005). "Treatment with laquinimod reduces development of active MRI lesions in relapsing MS". Neurology 64 (6): 987–91. doi:10.1212/01.WNL.0000154520.48391.69. PMID 15781813.
- ^ Darlington CL (2005). "Technology evaluation: NeuroVax, Immune Response Corp". Curr. Opin. Mol. Ther. 7 (6): 598–603. PMID 16370383.
- ^ a b Ristori, G; Buzzi MG, Sabatini U, Giugni E, Bastianello S, Viselli F, Buttinelli C, Ruggieri S, Colonnese C, Pozzilli C, Salvetti M (Oct 1999). "Use of Bacille Calmette-Guèrin (BCG) in multiple sclerosis". Neurology 53 (7): 1588–1589. PMID 10534275.
- ^ Paolillo, A; Buzzi MG, Giugni E, Sabatini U, Bastianello S, Pozzilli C, Salvetti M, Ristori G. (February 2003). "The effect of Bacille Calmette-Guérin on the evolution of new enhancing lesions to hypointense T1 lesions in relapsing remitting MS". J Neurol 250 (2): 247–248. doi:10.1007/s00415-003-0967-6. PMID 12622098.
- ^ Rutschmann, OT; McCrory, DC; Matchar, DB; Immunization Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines (Dec 2002). "Immunization and MS: a summary of published evidence and recommendations". Neurology 59 (12): 1837–1843. PMID 12499473.
- ^ Patel PN (2007). "Low-dose naltrexone for treatment of multiple sclerosis: clinical trials are needed". Ann Pharmacother 41 (9): 1549–50. doi:10.1345/aph.1H083. PMID 17623758.
- ^ Sriram S, Yao SY, Stratton C, Moses H, Narayana PA, Wolinsky JS (2005). "Pilot study to examine the effect of antibiotic therapy on MRI outcomes in RRMS". J. Neurol. Sci. 234 (1–2): 87–91. doi:10.1016/j.jns.2005.03.042. PMID 15935383.
- ^ Munger KL, Zhang SM, O'Reilly E, et al. (2004). "Vitamin D intake and incidence of multiple sclerosis". Neurology 62 (1): 60–5. PMID 14718698.
- ^ Elliott DE, Summers RW, Weinstock JV (2007). "Helminths as governors of immune-mediated inflammation". Int J Parasitol 37 (5): 457–64. doi:10.1016/j.ijpara.2006.12.009. PMID 17313951.
- ^ Zamboni P, Galeotti R (2010). "The chronic cerebrospinal venous insufficiency syndrome". Phlebology 25 (6): 269–79. doi:10.1258/phleb.2010.009083. PMID 21106999.
- ^ a b c d Namaka M, Crook A, Doupe A et al. (2008). "Examining the evidence: complementary adjunctive therapies for multiple sclerosis". Neurol Res 30 (7): 710–9. doi:10.1179/174313208X325038. PMID 18631428.
- ^ Farinotti M, Simi S, Di Pietrantonj C, et al. (2007). Farinotti, Mariangela. ed. "Dietary interventions for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD004192. doi:10.1002/14651858.CD004192.pub2. PMID 17253500.
- ^ Swank RL (1991). "Multiple sclerosis: fat-oil relationship". Nutrition (Burbank, Los Angeles County, Calif.) 7 (5): 368–76. PMID 1804476.
- ^ Swank RL, Goodwin J (2003). "Review of MS patient survival on a Swank low saturated fat diet". Nutrition (Burbank, Los Angeles County, Calif.) 19 (2): 161–2. doi:10.1016/S0899-9007(02)00851-1. PMID 12591551.
- ^ Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E (2006). "Cannabis use in patients with multiple sclerosis". Mult. Scler. 12 (5): 646–51. doi:10.1177/1352458506070947. PMID 17086912.
- ^ Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, Nunn AJ, Teare LJ, Fox PJ, Thompson AJ (2005). "Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up". J. Neurol. Neurosurg. Psychiatr. 76 (12): 1664–9. doi:10.1136/jnnp.2005.070136. PMC 1739436. PMID 16291891. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1739436.
- ^ Bohuslavizki KH, Hänsel W, Kneip A, Koppenhöfer E, Reimers A (October 1992). "Potassium channel blockers from Ruta--a new approach for the treatment of multiple sclerosis". Gen. Physiol. Biophys. 11 (5): 507–12. PMID 1291451.
- ^ Bodendiek SB, Mahieux C, Hänsel W, Wulff H (November 2008). "4-Phenoxybutoxy-substituted Heterocycles - a Structure-Activity Relationship Study of Blockers of the Lymphocyte Potassium Channel Kv1.3". Eur J Med Chem 44 (5): 1838–52. doi:10.1016/j.ejmech.2008.10.033. PMC 2662044. PMID 19056148. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2662044.
- ^ Beeton C, Chandy KG (December 2005). "Potassium channels, memory T cells, and multiple sclerosis". Neuroscientist 11 (6): 550–62. doi:10.1177/1073858405278016. PMID 16282596.
- ^ Wulff H, Pennington M (July 2007). "Targeting effector memory T-cells with Kv1.3 blockers". Curr Opin Drug Discov Devel 10 (4): 438–45. PMID 17659485.
- ^ Bennett M, Heard R (2004). Bennett, Michael H. ed. "Hyperbaric oxygen therapy for multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD003057. doi:10.1002/14651858.CD003057.pub2. PMID 14974004.
- ^ Oken BS, Kishiyama S, Zajdel D, et al. (2004). "Randomized controlled trial of yoga and exercise in multiple sclerosis". Neurology 62 (11): 2058–64. PMID 15184614.
- ^ Wang C, Collet JP, Lau J (2004). "The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review". Arch Intern Med 164 (5): 493–501. doi:10.1001/archinte.164.5.493. PMID 15006825.
See also
Listen to this article (info/dl)
This audio file was created from a revision of Treatment of multiple sclerosis dated 2008-01-25, and does not reflect subsequent edits to the article. (Audio help)More spoken articlesMultiple sclerosis and other demyelinating diseases of CNS (G35–G37, 340–341) Signs and symptoms Ataxia · Depression · Diplopia · Dysarthria · Dysphagia · Fatigue · Incontinence · Neurological fatigue · Nystagmus · Optic neuritis · Pain · Uhthoff's phenomenon · Dawson's fingersDiagnosis and evolution following Investigation Treatment Borderline forms Acute disseminated encephalomyelitis · Balo concentric sclerosis · Neuromyelitis optica · Marburg multiple sclerosis · Schilder's disease · Tumefactive multiple sclerosis
(Guillain-Barré syndrome and CIDP are PNS)Other Categories:- Autoimmune diseases
- Multiple sclerosis
- Medical treatments
- Neurological disorders
Wikimedia Foundation. 2010.