- Adderall
-
Adderall 
Combination of Dextroamphetamine Psychostimulant Levoamphetamine Psychostimulant Clinical data Trade names Dexedrine AHFS/Drugs.com monograph MedlinePlus a605027 Licence data US Daily Med:link Pregnancy cat. C(US) Legal status Schedule II (US) Dependence liability High Routes (Medical) Oral, (Recreational) Oral, Insufflated, Intravenous Identifiers CAS number 51-64-9  300-62-9
300-62-9ATC code N06BA02 N06BA01 PubChem CID 5826 DrugBank DB01576 ChemSpider 2900 
KEGG D03740 
ChEBI CHEBI:2679 
ChEMBL CHEMBL405 
 (what is this?) (verify)
(what is this?) (verify)Adderall is a brand name of amphetamine salts–based medication used for attention-deficit hyperactivity disorder and narcolepsy.[1] It is a brand-name psychostimulant medication composed of racemic amphetamine aspartate monohydrate, racemic amphetamine sulfate, dextroamphetamine saccharide, and dextroamphetamine sulfate, which are all amphetamine salts. It is thought to work by increasing the amount of dopamine and norepinephrine in the brain. In addition, the drug also acts as a potent dopamine reuptake inhibitor and norepinephrine reuptake inhibitor.[2] It is available in two formulations: IR (Instant Release) and XR (Extended Release). The immediate release formulation is indicated for use in Attention Deficit Hyperactivity Disorder (ADHD) and narcolepsy,[3] while the XR formulation is approved for use only with ADHD.[2]
Like other stimulant prescription drugs, Adderall directly affects the mesolimbic reward pathway in the brain. Amphetamine salts preparations are considered to have high abuse potential, and it is classified as Schedule II by the US DEA.
Contents
Medical uses
Adderall is used for attention-deficit hyperactivity disorder and narcolepsy.[1] It has been used to treat obesity, but The American Society of Health-System Pharmacists does not recommend this use.[1]
Attention deficit hyperactivity disorder
Adderall has been shown to significantly reduce symptoms associated with attention-deficit hyperactivity disorder (ADHD), and in many patients it presents minimal side-effects. Depending on dosage, the beneficial effects of stimulant medications can last several hours, allowing improved performance throughout the day.[4] Compared to the similar medication methylphenidate (sold under the brand name Ritalin and others), studies have suggested that Adderall is slightly more potent and has a longer period of efficacy, especially at lower doses.[5] For those who experience adverse side-effects to Ritalin or for whom Ritalin has become ineffective, Adderall is often recommended as a substitute.[6][7] For adolescents and adults, there is not adequate evidence that doses greater than 20 mg/day confer additional benefit, [8](p4) although higher doses may be necessary due to variations in body weight.[citation needed]
Other
Apart from the FDA-approved indications for the treatment of ADHD and narcolepsy, Adderall has also been used off-label to manage cases of treatment-resistant depression and exogenous obesity.[9]
Dosing and administration
Adderall is marketed as either an immediate-release tablet Adderall, or an extended-release capsule Adderall XR.[3]
Adderall XR utilizes the Microtrol extended-release delivery system, incorporating two types of beads. The first dissolves immediately, releasing half of the medication, while the second type dissolves much more slowly, releasing the remaining medication four hours later. Maximum plasma concentration is achieved in seven hours, compared to instant-release Adderall, which reaches maximum plasma concentration within three hours. As a result of its high bioavailability, Adderall XR's effectiveness is not altered by food absorption in the gastrointestinal tract. However, mean plasma concentration is prolonged by 2.5 hours (using a 900-calorie standard high-fat meal as the control). Medications that alter urinary pH will cause variations in the amount and method of excretion, and usage should be monitored when taken concurrently with Adderall.[10]
Manufacturer's claims of instant release have been disputed. A US patent granted for Adderall[11] was a pharmaceutical composition patent listing a rapid immediate-release oral dosage form. No claim of increased or smooth drug delivery was made. A recent double-blind, placebo-controlled crossover study conducted among 35 children ages 5–12 indicated that patients behaved similarly to those having taken other immediate-release amphetamines. The authors found that sustained-release dexamphetamine (the main isomeric-amphetamine component of Adderall) had a longer duration of action, however D-amphetamine was less effective in the first few hours.[12]
Adverse effects
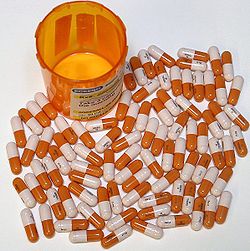 Adderall (mixed amphetamine salts) 25 mg XR
Adderall (mixed amphetamine salts) 25 mg XR See also: Amphetamine effects and dextroamphetamine effects
See also: Amphetamine effects and dextroamphetamine effects- Because Adderall may increase blood pressure, it carries the same risk of sudden death, stroke, and heart attack, as do methylphenidate and other stimulants used to treat ADHD, as well as the same risk of seizures in patients with a history of seizures.[2][13] Studies of long-term use of Adderall and methylphenidate in children have shown a temporary decrease in growth rate that does not affect final adult height.[14] Stimulant medications also decrease appetite in some people, leading to weight loss, and this effect is more common with Adderall than methylphenidate[14] or atomoxetine. Changes in vision have been reported with both Adderall and methylphenidate.[2][13] Some patients, children and adolescents, may show signs of increased aggression.[2]
Like all amphetamines that increase norepinephrine levels, it may cause akathisia or motor restlessness.[15] Women who are pregnant should avoid taking Adderall, especially during early pregnancy. Studies on rats show long-term neurological and behavioral changes resulting from prenatal and early postnatal exposure to amphetamines.[2]
Contraindications, interactions, and precautions
The following provides only general guidelines and is not comprehensive. Please refer to a more comprehensive list for further information regarding co-administration of amphetamine with other substances.
- MAOIs (monoamine oxidase inhibitors, e.g., phenelzine, selegiline, iproniazid, etc.) — Do not administer amphetamine for a minimum of two weeks after last use of MAOI type drug. High risk for hypertensive crisis. Preliminary trials of low-dose amphetamine and MAOIs being administered together are in progress. However, this is to be done only under strict supervision of the prescribing parties.
- SSRIs (selective serotonin reuptake inhibitors, e.g., fluvoxamine, citalopram, paroxetine, etc.) — While a common combination, and although rare, the risk for serotonin syndrome exists. (Use only when directed)
- NRIs (norepinephrine reuptake inhibitors, e.g., atomoxetine, etc.) — NRI medications and amphetamine both enhance noradrenergic activity. Possible augmentation/potentiation of effects. (Use only when directed)
- SNRIs (selective serotonin-norepinephrine reuptake inhibitors) — See SSRIs and NRIs.
- Bupropion — Both bupropion and amphetamine have noradrenergic and dopaminergic activity. Possible augmentation/potentiation of effects. Bupropion has pro-convulsant properties that may be enhanced or cumulatively potentiated by amphetamine.[16] (Use only when directed)
- Tricyclics and related compounds (tricyclic antidepressant) — See SNRIs and SSRIs. Possible potentiation of serotonin-, dopamine-, and norepinephrine-related drug effects. The combination of tricyclic and amphetamine compounds/other direct-acting sympathomimetics has been associated with increased sympathetic action. Adjustments to dose may be required. Concurrent use not generally recommended due to interaction between direct-acting sympathomimetics such as amphetamine and tricyclics. Indirect-acting sympathomimetics may have decreased efficacy when combined with tricyclics (tricyclic blockade may inhibit the action of some indirect-acting sympathomimetics).
- CYP2D6 (liver enzyme) inhibitors, e.g., most SSRIs such as fluoxetine, citalopram, paroxetine, etc. Some anti-psychotics such as thioridazine, haloperidol, and levomepromazine. The stimulant cocaine. The opioid agonist methadone. There are additional drugs that inhibit CYP2D6. It is important to determine if any medication or drug taken is a CYP2D6 inhibitor. Taking a CYP2D6-inhibiting drug along with amphetamine will lead to an elevated level of amphetamine in the system, resulting in the drug's remaining in the body for a longer period, which can lead to undesirable and possibly serious side effects.
Pregnancy
FDA pregnancy category C. It is not known whether Adderall will harm an unborn baby. It could cause premature birth, low birth weight, or withdrawal symptoms in a newborn if the mother takes this medication during pregnancy. Tell your doctor if you are pregnant or plan to become pregnant while using this medication.[17]
Government warnings
On February 9, 2005, Health Canada suspended all sales of Adderall XR after data collected by manufacturer Shire Pharmaceuticals linked the drug to 12 sudden deaths in American children.[18] Further research found data suggesting use of Adderall resulted in an increased risk of cardiac defect. Although more than 37 million prescriptions for Adderall were filled during the four years prior, the U.S. Food and Drug Administration could find no increased risk of sudden death among Adderall users.[18][19] In August 2005, Health Canada followed the committee report of three independent physicians and lifted the ban on Adderall XR.[20][21] Given that persons with ADHD are more likely to engage in risky or dangerous behavior, it has been suggested that stimulant medications for persons with ADHD may actually result in lower incidence of premature death.[22] The use of Adderall is generally not advised in those persons with pre-existing cardiac or mental illnesses. It is also not advised in persons who have a history of drug abuse.[3] Although FDA safety advisors voted 8 to 7 to issue a black box warning, the FDA's pediatric advisory committee refused to give the drug its most severe black box warning in March 2006.[23] A Black Box Warning regarding amphetamine abuse potential is in place, however. In September 2008, Britain's National Institute for Health and Clinical Excellence urged physicians not to prescribe Adderall or similar drugs to children under 5, and to exhaustively consider other approaches to behavioral modification before prescribing such drugs to children 5 and up.[24]
Prolonged use
Prolonged high doses of amphetamines followed by an abrupt cessation can result in extreme fatigue, insomnia, irritability, and mental depression. Chronic abuse of amphetamines can result in the manifestation of amphetamine psychosis.[2]
Chemistry
Adderall's effects are similar to other CNS stimulants of the same class and preparation. (See amphetamine for details.)
Urinary and stomach pH levels can have a strong effect on DL-amphetamine excretion and absorption.[25] An acidic stomach and GI pH will decrease the absorption of Adderall,[10] and acidic urine levels will decrease the reabsorption of the drug through the renal system.[26] Co-administration of acidic substances (e.g., citric acid) causes decreased renal reabsorption of DL-amphetamine; whereas, alkaline agents (e.g., antacids) may cause a marked increase in renal tubular reabsorption. The increased reabsorption can increase the retention of amphetamines, with potential to result in dangerously high serum levels.[26]
Adderall XR
Adderall XR consists of the following amphetamine compounds in equal proportions: dextroamphetamine saccharate, dextroamphetamine sulfate, racemic amphetamine aspartate monohydrate, and racemic amphetamine sulfate.[2] Breakdown rates are affected by many factors including urinary and stomach pH,[10][25] weight, gender, other medications being taken, and age. Alkalinity increases bioavailability, while acidity causes the drug to be excreted more quickly. Manufacturers claim that the mixture of salts in Adderall XR makes its effects smoother (that is, makes softer highs and lows).[27] The mixture of salts and their ratios are as follows:
- 1/4 dextroamphetamine saccharate
- 1/4 dextroamphetamine sulfate
- 1/4 (racemic dextro/levo-)amphetamine aspartate monohydrate
- 1/4 (racemic dextro/levo-)amphetamine sulfate
These four salts are metabolized at different rates and possess diverse half lives,[citation needed] thereby resulting in a less dramatic onset and termination of therapeutic action, as compared to single-salt amphetamine preparations.[citation needed]
Metabolism
"The mean elimination half-life for d-amphetamine is 10 hours in adults; 11 hours in adolescents aged 13–17 years and weighing less than or equal to 75 kg/165 lbs; and 9 hours in children aged 6 to 12 years. For the l-amphetamine, the mean elimination half-life in adults is 13 hours; 13 to 14 hours in adolescents; and 11 hours in children aged 6 to 12 years. On a mg/kg body weight basis children have a higher clearance than adolescents or adults."[8](p2)
Generic forms
Both Adderall IR and Adderall XR are available in generic forms.[28][29][30] The generic version of Adderall IR is available as generic drugs but the generic version of Adderall XR is only available as authorized generics.[31][32] Authorized generics are still manufactured by the brand name manufacturer but marketed and sold by a different company. Authorized generics are exactly the same as the brand name product both in active and inactive ingredients—they go through exactly the same brand manufacturing line, yet different labels are put on at the end of the manufacturing process.
Mechanism of action
Main article: Dextroamphetamine mechanism of actionWith respect to central stimulant actions, the S(+) isomer (i.e., dextroamphetamine) is several times more potent than its R(-)enantiomer (i.e., levoamphetamine); this is not necessarily the case with other actions produced by amphetamine, in particular those produced in the periphery such as its cardiovascular actions.[33] Dextroamphetamine induces more euphoria, whereas levoamphetamine induces more depression. The overall greater potency of the dextro form to central actions suggests that this form may have a higher potential for abuse.[34]
Adderall’s inclusion of levoamphetamine provides the pharmaceutical with a quicker onset and longer clinical effect compared to pharmaceuticals formulated exclusively of dextroamphetamine.[35] Although it seems the human brain has a preference for dextroamphetamine over levoamphetamine, it has been reported that certain children have a better clinical response to levoamphetamine.[36] Amphetamines are believed to exert their effects by binding to the monoamine transporters and increasing extracellular levels of the biogenic amines dopamine, norepinephrine, and serotonin[citation needed] .
It is hypothesized that D-amphetamine acts primarily on the dopaminergic (DA) systems, while L-amphetamine is comparatively norepinephrinergic (NE)[citation needed]. The primary reinforcing and behavioral-stimulant effects of amphetamine, however, are linked to enhanced dopaminergic activity, primarily in the mesolimbic dopaminergic pathway. Amphetamine binds to the dopamine transporter (DAT) and blocks the transporter's ability to clear DA from the synaptic space. In addition, amphetamine is transported into the cell, which leads to dopamine efflux (DA is transported out of the cell and into the synaptic space via reverse transport of the DAT).
Amphetamine also possesses the ability to inhibit the enzymes monoamine oxidase-A and -B (MAO-A and MAO-B) in high doses. MAO-A is responsible for the breakdown of serotonin, dopamine, norepinephrine, and epinephrine. MAO-B is responsible for breaking down dopamine (more potently than MAO-A) and phenylethylamine (PEA), which has actions similar to those of amphetamine itself, and is thought to be involved in feelings of lust, confidence, obsession, and sexuality. Some of the first antidepressants successfully marketed are, in fact, Monoamine-Oxidase inhibitors. However, MAO inhibition seen with amphetamine is not substantial enough in duration and quantity to entail the need for a tyramine-limited diet, unlike the more potent and long-lived MAO-inhibiting antidepressants.
Amphetamine's ability to cause the inhibition of MAO results in the accumulation of monoamines: Amphetamine directly stimulates the release of these neurochemicals, resulting in a potent elevation in monoamine neurotransmission. In sum, the effect of amphetamines is to increase neurotransmitter availability in the synapse, by both releasing more neurotransmitters and prolonging their availability in the synapse by slowing their removal.
Performance-enhancing use
Adderall is widely used as a "study drug" at many universities,[37] due to Adderall's reported ability to help focus energy and concentration to a much higher level than normal.[citation needed] It enables the user to focus and stay awake. Stories of students writing papers continuously for an unusually long time or "cramming" all night for an exam with no loss of energy or concentration are common.[37] College campuses known to be highly competitive or have a high rate of binge drinking had up to 25% of students use an ADHD medication within one year, a survey of students at 119 colleges across the country concluded.[citation needed]
Many athletic organizations have restricted the usage of Adderall by athletes. The NCAA has banned the use of Adderall for its collegiate athletes without a prescription and adequate records of evaluation and diagnosis of ADHD.[38] Nevada State Athletic Commission has also banned athletes in the state from using Adderall. Tim Credeur was removed from a UFC fight on the finale of The Ultimate Fighter 7 because of a positive drug test due to his use of it. In the National Football League, New Orleans Saints kicker Garrett Hartley served a four-game suspension when the 2009 NFL regular season began because he tested positive for the banned stimulant. The Arizona Cardinals tight end Ben Patrick received a four-game suspension as a result of using Adderall.[39]
Detection of use
Amphetamine is frequently measured in hair, oral fluid, sweat, or urine as part of a drug abuse testing program. Techniques such as immunoassay may cross-react with a number of sympathomimetics drugs, so chromatographic methods specific for amphetamine should be employed to prevent false-positive results. Chiral techniques may be employed to help distinguish the source of the drug, whether obtained legally (by prescription) or illegally or possibly as a result of formation from a prodrug such as lisdexamfetamine or selegiline. Chiral separation can be used to differentiate Adderall use from use of another prescription form of amphetamine or from use of illicit amphetamine, since Adderall is unique in having a 3:1 mixture of the d- and l-isomers.[40][41][42]
History
Adderall is available as an instant-release (IR) and an extended-release (XR) drug. Adderall instant-release is manufactured today by Teva and Barr Pharmaceuticals. Shire Pharmaceuticals, the creator of Adderall IR, no longer produces it. However, Shire does continue to manufacture the extended-release version of Adderall ("Adderall XR"). Shire introduced the Adderall brand in 1996 in the form of a multi-dose, instant-release tablet derived from an original formula of the weight management drug Obetrol. In 2006, Shire agreed to sell rights to the Adderall name for this instant-release medication to Duramed Pharmaceuticals[43] DuraMed Pharmaceuticals was acquired by Teva Pharmaceuticals in 2008 when Teva completed its acquisition of Barr Pharmaceuticals (including Barr's Duramed division).[44] Therefore, following its acquisition of Duramed, Teva is in the somewhat unusual position of manufacturing both a generic formulation of Adderall instant-release (under its Barr Division) as well as "brand name" Adderall (under its DuraMed division.)
Reflecting the change in manufacturers from Shire Pharmaceuticals to Teva's DuraMed Pharmaceuticals, the "imprint" on Adderall instant-release tablets has changed. One side of the instant-release tablets now bears the DuraMed Pharmaceuticals imprint, a lowercase letter "d" over a lowercase letter "p". This is a change from the previous uppercase "AD" imprint on the tablets.
The active ingredients of Adderall include a combination of dextroamphetamine and racemic DL-amphetamine salts.[45] In 2001, Shire introduced an extended-release preparation of these ingredients in a variety of dosages under the brand name "Adderall XR," on which Shire retains exclusive patent rights until the patent expires, expected in 2018.[46] However, due to issues with Shire's inability to evergreen (extend the patent life by obtaining either a new FDA indication or application of other patent life) the patent for Adderall XR, the drug has become available in a generic form.[47] In 2009, Barr and Shire reached a settlement agreement permitting Barr to offer a generic form of the drug beginning April 1, 2009.[48]
Legal status
- Japan: Any medicine containing Methamphetamine or Amphetamine is defined as one of “Prohibited Stimulants” and strictly restricted in Japan. Nobody can bring any medicine containing Methamphetamine or Amphetamine (Adderall and so on) into Japan.[49]
- South Korea: Non-prescribed Amphetamine-based medications are banned in South Korea. They are illegal to import without a doctor's prescription.[50] Adderall is not currently prescribed inside Korea, but other stimulant medications such as Ritalin are.
- Thailand: Amphetamine and dextroamphetamine are classified as Type 1 Narcotics.[51]
References
- ^ a b c "Adderall". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/adderall.html. Retrieved 3 April 2011.
- ^ a b c d e f g h "Adderall XR prescribing information". Shire US. March 2009. http://pi.shirecontent.com/PI/PDFs/AdderallXR_USA_ENG.PDF. Retrieved 2009-06-23.
- ^ a b c "ADDERALL (CII)" (PDF). Food and Drug Administration. March 2007. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/011522s040lbl.pdf. Retrieved 2009-06-23.
- ^ Biederman J, Lopez FA, Boellner SW, Chandler MC (August 2002). "A randomized, double-blind, placebo-controlled, parallel-group study of SLI381 (Adderall XR) in children with attention-deficit/hyperactivity disorder". Pediatricsa 110 (2 Pt 1): 258–66. doi:10.1542/peds.110.2.258. PMID 12165576. http://pediatrics.aappublications.org/cgi/content/full/110/2/258. Retrieved 2009-06-23.
- ^ "Adderall compared to methylphenidate; Efficacy and safety
- ^ Pelham WE, Aronoff HR, Midlam JK, et al. (April 1999). "A comparison of Ritalin and Adderall: efficacy and time-course in children with attention-deficit/hyperactivity disorder". Pediatrics 103 (4): e43. doi:10.1542/peds.103.4.e43. PMID 10103335. http://pediatrics.aappublications.org/cgi/content/full/103/4/e43. Retrieved 2009-06-23.
- ^ Pliszka SR, Browne RG, Olvera RL, Wynne SK (May 2000). "A double-blind, placebo-controlled study of Adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder". Journal of the American Academy of Child and Adolescent Psychiatry 39 (5): 619–26. doi:10.1097/00004583-200005000-00016. PMID 10802980.
- ^ a b FDA (2007). Medication guide adderall xr. pp. 1–14. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021303s015lbl.pdf. Retrieved 2011-02-04.
- ^ Fernstrom, Madelyn (2006-10-26). "Quick-fix diet drugs: Effective or harmful?". Today (Msnbc). http://www.msnbc.msn.com/id/15385195/. Retrieved 2009-06-23.
- ^ a b c Aschenbrenner, Diane S.; Samantha J. Venable (2008). Drug Therapy in Nursing. Lippincott Williams & Wilkins. p. 359. ISBN 0-7817-6587-0.
- ^ US patent 6384020, Flanner, Henry H., et al., "A pharmaceutical composition comprising lactitol and one or more amphetamine salts in a rapid-release formulation", issued 2002-05-07
- ^ Buck, ML (March 2002). "Amphetamines in the Treatment of Attention-Deficit/Hyperactivity Disorder" (PDF). Pediatric Pharmacotherapy 8 (3). http://www.healthsystem.virginia.edu/alive/pediatrics/PharmNews/200203.pdf. Retrieved 2009-06-23.
- ^ a b "Ritalin prescribing information" (PDF). Novartis. April 2007. http://www.pharma.us.novartis.com/product/pi/pdf/ritalin_ritalin-sr.pdf. Retrieved 2009-06-23.
- ^ a b Pliszka SR, Matthews TL, Braslow KJ, Watson MA (May 2006). "Comparative effects of methylphenidate and mixed salts amphetamine on height and weight in children with attention-deficit/hyperactivity disorder". Journal of the American Academy of Child and Adolescent Psychiatry 45 (5): 520–6. doi:10.1097/01.chi.0000205702.48324.fd. PMID 16670648.
- ^ "ADHD Medications - Actions and Side Effects". Healthcenter.pterrywave.com. http://healthcenter.pterrywave.com/ADHD/Drugs.aspx. Retrieved 2011-10-31.
- ^ "A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor". Ncbi. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC514842/. Retrieved 2011-10-31.
- ^ "Adderall, Prescription Marketed Drugs, www.drugsdb.eu". http://drugsdb.eu/drug.php?d=Adderall&m=Barr%20Laboratories%20Inc.&id=d16d512f-48e9-400e-8ac1-7cbd8dd61dc1.xml.
- ^ a b "Public Health Advisory for Adderall and Adderall XR". Food and Drug Administration. 2009-04-30. http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm051672.htm. Retrieved 2009-06-23.[dead link]
- ^ DeNoon, Daniel (2005-02-10). "Sudden Death in 12 Kids on ADHD Drug Adderall". WebMD. http://www.webmd.com/add-adhd/news/20050210/sudden-death-in-12-kids-on-adhd-drug-adderall-news. Retrieved 2009-06-23.
- ^ "Report of the Adderall XR New Drug Committee". Health Canada. 2005-08-26. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/new-drug-com-nouvelle-drogue/ndca_rep_cnma_rap_2005-08-25-eng.php. Retrieved 2009-06-23.
- ^ Rosack, J (7 October 2005). "Canada reverses ban on ADHD medication". Psychiatric News (American Psychiatric Association) 40 (19): 2. ISSN 1559-1255. http://pn.psychiatryonline.org/cgi/content/full/40/19/2. Retrieved 2009-06-23.
- ^ Brown, Thomas (May 2006). "Excessive Fears about Stimulant Medications for ADHD". The BrownLetter on ADD. http://www.drthomasebrown.com/resources/newsletters/may06.html. Retrieved 2009-06-23.
- ^ "Dire warning not urged for ADHD drugs". Washington Post. 2006-03-23. http://www.washingtonpost.com/wp-dyn/content/article/2006/03/22/AR2006032202079.html. Retrieved 2009-06-23.
- ^ Cummings, Denis (2008-09-26). "British Health Agency Discourages Ritalin Use". FindingDulcinea. http://www.findingdulcinea.com/news/health/September-October-08/British-Health-Agency-Discourages-Ritalin-Use.html. Retrieved 2009-06-23.
- ^ a b Washington, Neena (1991). Antacids and Anti-reflux Agents. CRC Press. p. 175. ISBN 0-8493-5444-7. http://books.google.com/?id=MKxtXgtt5SIC.
- ^ a b Nobles, Sylvia Tindell (2001). Delmar's Drug Reference for Health Care Professionals. Cengage Learning. p. 10. ISBN 0-7668-2523-X.
- ^ Reynolds, Cecil R; Fletcher-Janzen, Elaine (2009). Handbook of Clinical Child Neuropsychology (3rd, revised ed.). Springer. p. 531. ISBN 978-0-387-70708-2. http://books.google.com/?id=Cm86pbWGVj8C. Retrieved 2009-06-23.
- ^ "Adderall IR; Adderall XR: Are they from Shire, Barr, Teva, or Ranbaxy?". Armand Rossetti. InjuryBoard.com. 2009-08-17. http://westpalmbeach.injuryboard.com/fda-and-prescription-drugs/adderall-ir-adderall-xr-are-they-from-shire-barr-teva-or-ranbaxy.aspx?googleid=269158. Retrieved 2009-10-01.
- ^ "Has anyone noticed change in Adderall (brand, not generic) formula?". addforums. http://www.addforums.com.+2009-08-26. http://www.addforums.com/forums/showthread.php?t=56460. Retrieved 2009-10-01.
- ^ "Why has brand-name Adderall changed formula, and now has 'dp' imprint, rather than 'AD'?". ADHDCentral.com. http://www.healthcentral.com.+2009-08-17. http://www.healthcentral.com/adhd/c/question/513341/59075. Retrieved 2009-02-11.
- ^ http://blue.regence.com/trgmedpol/drugs/dru179.pdf
- ^ "FDA Listing of Authorized Generics" (PDF). http://www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/UCM183605.pdf. Retrieved 2011-10-31.
- ^ Williams, David A; Lemke, Thomas L; Foye, William O, eds (2002). "Hallucinogens, stimulants, and related drugs of abuse". Foye's principles of medicinal chemistry (5th, illustrated ed.). Lippincott Williams & Wilkins. p. 445. ISBN 978-0-683-30737-5. http://books.google.com.au/books?id=KGLF4ZudTyAC&pg=PA445&lpg=PA445&dq=caffiene+is+works+in+the+same+way+as+amphetamine+only+less+potent&source=bl&ots=6y9FDsWGPB&sig=v9HY-NwUzxzbEqs30oBkcemDipU&hl=en&ei=b2_IScTmMNeGkAXuu-joAg&sa=X&oi=book_result&resnum=1&ct=result. Retrieved 2009-06-23.
- ^ edited by Alan F. Schatzberg, Charles B. Nemeroff. (2004). Schatzberg, Alan F; Nemeroff, Charles B. eds. The American Psychiatric Publishing textbook of psychopharmacology (3rd ed.). American Psychiatric Publishing. ISBN 978-1-58562-060-9. http://books.google.com/?id=5QfL19H5nVkC. Retrieved 2009-06-23.
- ^ Glaser PE, Thomas TC, Joyce BM, Castellanos FX, Gerhardt GA (March 2005). "Differential effects of amphetamine isomers on dopamine release in the rat striatum and nucleus accumbens core". Psychopharmacology 178 (2–3): 250–8. doi:10.1007/s00213-004-2012-6. PMID 15719230.
- ^ Arnold (2000). "Methylphenidate vs Amphetamine: Comparative Review". Journal of Attention Disorders 3 (4): 200–211. doi:10.1177/108705470000300403.
- ^ a b Kristina M. Hall et al., "Illicit Use of Prescribed Stimulant Medication Among College Students," Journal of American College Health, Vol. 53, No. 4 (2005).
- ^ "NCAA Banned Drugs and Medical Exceptions Policy". http://www.ncaa.org/wps/portal/ncaahome/ncaa/NCAA/Legislation+and+Governance/Eligibility+and+Recruiting/Drug+Testing/Medical+Exceptions+Policy+Video. Retrieved 27 June 2011.
- ^ "Patrick tests positive for amphetamine". Associated Press. ESPN. 2009-06-15. http://sports.espn.go.com/nfl/news/story?id=4261608. Retrieved 2009-06-23.
- ^ JT Cody, S Valtier, SL Nelson. Amphetamine excretion profile following multidose administration of mixed salt amphetamine preparation. J. Anal. Toxicol. 28: 563–573, 2004.
- ^ Paul BD, Jemionek J, Lesser D, Jacobs A, Searles DA. Enantiomeric separation and quantitation of (+/-)-amphetamine, (+/-)-methamphetamine, (+/-)-MDA, (+/-)-MDMA, and (+/-)-MDEA in urine specimens by GC-EI-MS after derivatization with (R)-(-)- or (S)-(+)-alpha-methoxy-alpha-(trifluoromethy)phenylacetyl chloride (MTPA). J. Anal. Toxicol. 28: 449–455, 2004.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp. 85–88.
- ^ "Barr and Shire Sign Three Agreements" (Press release). Barr Pharmaceuticals. 2006-08-14. http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/08-14-2006/0004416042. Retrieved 2009-06-23.
- ^ "Teva Completes Acquisition of Barr". Drugs.com. http://www.drugs.com/news/teva-completes-acquisition-barr-15356.html. Retrieved 2011-10-31.
- ^ Adderall (Amphetamine Mixed Salts) drug description – U.S. Food and Drug Administration (FDA) approved labeling for prescription drugs and medications at RxList
- ^ "Shire’s Adderall XRTM receives patent protection" (PDF) (Press release). Shire Pharmaceuticals. 2001-11-28. http://www.shire.com/shire/uploads/press/shire/28112001B.pdf. Retrieved 2009-06-23.[dead link]
- ^ Foley, Stephen (2006-08-16). "Shire in deal with Barr to delay launch of rival to its ADHD drug". London: The Independent. http://www.independent.co.uk/news/business/news/shire-in-deal-with-barr-to-delay-launch-of-rival-to-its-adhd-drug-412102.html. Retrieved 2006-06-23.
- ^ "Teva sells 1st generic of Adderall XL in US". Associated Press. Forbes Magazine. 2009-04-02. http://www.forbes.com/feeds/ap/2009/04/02/ap6248888.html. Retrieved 2009-04-22.[dead link]
- ^ http://kouseikyoku.mhlw.go.jp/kantoshinetsu/gyomu/bu_ka/shido_kansa/documents/qa_bringmedicines_070618.pdf
- ^ "Korean Customs Service : Restricted Items". English.customs.go.kr. http://english.customs.go.kr/kcsweb/user.tdf?a=common.HtmlApp&c=1501&page=/english/html/kor/personal/personal_01_04.html&mc=ENGLISH_PERSONAL_TRAVELERS_040. Retrieved 2011-10-31.
- ^ "Erowid Psychoactive Vaults : Thailand Law". Erowid.org. 2009-03-27. http://www.erowid.org/psychoactives/law/countries/law_thailand.shtml. Retrieved 2011-10-31.
Phenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolPsychostimulants, agents used for ADHD, and nootropics (N06B) Centrally acting sympathomimetics Xanthine derivatives Glutamate receptor CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • S-18986 • Sunifiram • UnifiramEugeroics / Benzhydryl compounds Histamine H3 receptor antagonists GABAA α5 inverse agonists Dopamine D1 receptor agonists α7 nicotinic agonists / PAMs AR-R17779 • PNU-282,987 • SSR-180,711Prolyl endopeptidase inhibitors S-17092Alpha-adrenergic agonists Other psychostimulants and nootropics Acetylcarnitine • Adafenoxate • Bifemelane • Carbenoxolone • Citicoline • Cyprodenate • Ensaculin • Idebenone • Ispronicline • Deanol • Dimebon • Fipexide • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • P7C3 • Pirisudanol • Pyritinol • Rubidium • Sulbutiamine • Taltirelin • Tricyanoaminopropene • VinpocetineStimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic amines Categories:- Amphetamines
- Nootropics
- Combination drugs
Wikimedia Foundation. 2010.