- Overman rearrangement
-
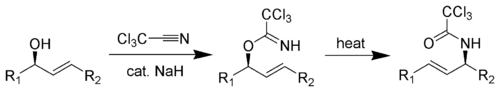
The Overman rearrangement is a chemical reaction that can be described as a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate.[1][2][3] The Overman rearrangement was discovered in 1974 by Larry Overman.[4]
The [3,3]-sigmatropic rearrangement is diastereoselective and can be catalyzed by heat, Hg(II), or Pd(II).[5] The resulting allylamine structures can be transformed into many chemically and biologically important natural and un-natural amino acids (like (1-adamantyl)glycine).[6]
The Overman rearrangement may also be used for asymmetric synthesis.[7][8]
References
- ^ Larry E. Overman (1976). "A general method for the synthesis of amines by the rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions". J. Am. Chem. Soc. 98 (10): 2901–2910. doi:10.1021/ja00426a038.
- ^ Overman, L. E. (1980). "Allylic and propargylic imidic esters in organic synthesis". Accounts of Chemical Research 13 (7): 218–224. doi:10.1021/ar50151a005.
- ^ Organic Syntheses, Coll. Vol. 6, p.507; Vol. 58, p.4 (Article)
- ^ Overman, L. E. (1974). "Thermal and mercuric ion catalyzed [3,3]-sigmatropic rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions". Journal of the American Chemical Society 96 (2): 597–599. doi:10.1021/ja00809a054.
- ^ Overman, L. E.; Carpenter, N. E. Org. React. 2005, 66, 1. (doi: 10.1002/0471264180.or066.01)
- ^ Chen, Y. K.; Lurain, A. E.; Walsh, P. J. (2002). "A General, Highly Enantioselective Method for the Synthesis of D and L α-Amino Acids and Allylic Amines". Journal of the American Chemical Society 124 (41): 12225–12231. doi:10.1021/ja027271p.
- ^ Anderson, C. E.; Overman, L. E. J. Am. Chem. Soc. 2003, 125, 12412–12413. (doi:10.1021/ja037086r)
- ^ Asymmetric Overman Rearrangement Organic Syntheses, Vol. 82, p.134 (2005). (Article)
Further reading
- Isobe, M. et al. Tetrahedron Lett. 1990, 31, 3327.
- Allmendinger, T. et al. Tetrahedron Letters 1990, 31, 7301.
- Nishikawa, T.; Asai, M.; Ohyabu, N.; Isobe, M.; J. Org. Chem. 1998, 63(1), 188-192. (PMID: 11674062)
Categories:- Rearrangement reactions
- Name reactions
- Carboximidates
Wikimedia Foundation. 2010.