- Monooxygenase DBH-like 1
-
Monooxygenase, DBH-like 1 Identifiers Symbols MOXD1; DKFZp564G202; MOX; PRO5780; dJ248E1.1 External IDs OMIM: 609000 MGI: 1921582 HomoloGene: 22904 GeneCards: MOXD1 Gene EC number 1.14.17.1 Gene Ontology Molecular function • monooxygenase activity
• dopamine beta-monooxygenase activity
• copper ion binding
• metal ion bindingCellular component • endoplasmic reticulum
• endoplasmic reticulum membrane
• membrane
• integral to membraneBiological process • catecholamine metabolic process
• cellular processSources: Amigo / QuickGO RNA expression pattern 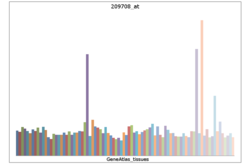
More reference expression data Protein domains 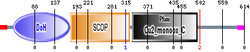
Orthologs Species Human Mouse Entrez 26002 59012 Ensembl ENSG00000079931 ENSMUSG00000020000 UniProt Q6UVY6 Q9CXI3 RefSeq (mRNA) NM_015529 NM_021509.5 RefSeq (protein) NP_056344 NP_067484.2 Location (UCSC) Chr 6:
132.62 – 132.72 MbChr 10:
23.94 – 24.02 MbPubMed search [1] [2] DBH-like monooxygenase protein 1, also known as monooxygenase X, is an enzyme that in humans is encoded by the MOXD1 gene.[1][2]
DBH-like 1 maintains many of the structural features of dopamine beta-monooxygenase DBH.[3] Since Peptidylglycine alpha-hydroxylating monooxygenase (PHM; EC 1.14.17.3) is homologous to dopamine beta-monooxygenase (DBM; EC 1.14.17.1)[4] this concerns a structural basis for a new family of copper type II, significantly specific for ascorbate-dependent monooxygenases[4] based on the corresponding mouse homolog.[2] The pathway of catecholamine synthesis is a possible catecholamine-binding metabolic copper[5] enzyme domain, a neuron-like property encoding MOX without a signal sequence enzyme metabolism resolving the monooxygenase X chemical pathway[5] of an unknown substrate,[2] exogenous MOX is not secreted, and it localizes throughout the endoplasmic reticulum,[5] in both endocrine or nonendocrine cells.[5]
Contents
Deficiency
DBH deficiency has been treated effectively with L-threo-3,4-dihydroxyphenylserine (DOPS).[6]
See also
- Dopamine-beta-hydroxylase-DBH,
- Dopamine beta-monooxygenase-DBM,
- Peptidylglycine alpha-hydroxylating monooxygenase-PHM
- peptidyl-alpha-hydroxyglycine alpha-amidating lyase-PAL
- Tyrosine 3-monooxygenase-TH.
References
- ^ "Entrez Gene: monooxygenase, DBH-like 1". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=26002.
- ^ a b c Chambers KJ, Tonkin LA, Chang E, Shelton DN, Linskens MH, Funk WD (September 1998). "Identification and cloning of a sequence homologue of dopamine beta-hydroxylase". Gene 218 (1-2): 111–20. doi:10.1016/S0378-1119(98)00344-8. PMID 9751809.
- ^ Prigge ST, Mains RE, Eipper BA, Amzel LM.. (August 2000). "New insights into copper monooxygenases and peptide amidation: structure, mechanism and function.". Cell Mol Life Sci. 57 (8-9): 1236–59. doi:10.1007/PL00000763. ISSN 1420682X. PMID 11028916.
- ^ a b Southan C, Kruse LI (September 1989). "Sequence similarity between dopamine beta-hydroxylase and peptide alpha-amidating enzyme: evidence for a conserved catalytic domain.". FEBS Lett. 255 (1): 116–20. doi:10.1016/0014-5793(89)81072-5. PMID 2792366.
- ^ a b c d Xin X, Mains RE, Eipper BA.. (November 2004). "Monooxygenase X, a member of the copper-dependent monooxygenase family localized to the endoplasmic reticulum.". J. Biol. Chem. 279 (46): 48159–67. doi:10.1074/jbc.M407486200. PMID 15337741.
- ^ Vincent S, Robertson D. (May 2002). "The broader view: catecholamine abnormalities.". Clin Auton Res. Suppl. 1: 144–9. ISSN 0959-9851. PMID 12102462.
Further reading
- Clark HF, Gurney AL, Abaya E, et al. (2003). "The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment.". Genome Res. 13 (10): 2265–70. doi:10.1101/gr.1293003. PMC 403697. PMID 12975309. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=403697.
- Xin X, Mains RE, Eipper BA (2004). "Monooxygenase X, a member of the copper-dependent monooxygenase family localized to the endoplasmic reticulum.". J. Biol. Chem. 279 (46): 48159–67. doi:10.1074/jbc.M407486200. PMID 15337741.
- Bon S, Lamouroux A, Vigny A, Massoulié J, Mallet J, Henry JP. (October 1991). "Amphiphilic and nonamphiphilic forms of bovine and human dopamine beta-hydroxylase.". J. Neurochem. 57 (4): 1100–11. doi:10.1111/j.1471-4159.1991.tb08267.x. ISSN 0022-3042. PMID 1654385. http://www.ebi.ac.uk/citexplore/citationDetails.do?externalId=1654385&dataSource=MED.
This EC 1.14 enzyme-related article is a stub. You can help Wikipedia by expanding it.
