Methylisopropyllysergamide
- Methylisopropyllysergamide
-
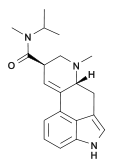
Methylisopropyllysergamide (lysergic acid methylisopropyl amide, MIPLA) is an analogue of LSD that was originally discovered by Albert Hofmann at Sandoz during the original structure-activity research into LSD. It has subsequently been investigated in more detail by the team led by David E. Nichols at Purdue University. Methylisopropyllysergamide is a structural isomer of LSD, with the alkyl groups on the amide nitrogen having been subjected to a methylene shuffle. MIPLA and its ethylisopropyl homologue are the only simple N,N-dialkyl lysergamides that approach the potency of LSD itself, being around 1/3-1/2 the potency of LSD,[1] while all other dialkyl analogues tested (dimethyl, dipropyl, methylethyl etc.) are only around 1/10 as potent as LSD,[2] although some N-monoalkyl lysergamides such as the sec-butyl and t-butyl derivatives were also found to show an activity profile and potency comparable to LSD,[3] and the mono-isopropyl derivative is only slightly weaker than MIPLA. Apart from its lower potency, the hallucinogenic effects of methylisopropyllysergamide are similar to those of LSD itself, and the main use for this drug has been in studies of the binding site at the 5-HT2A receptor through which LSD exerts most of its pharmacological effects.[4]
See also
- Lysergic acid 2-butyl amide
- Lysergic acid 3-pentyl amide
References
- ^ Huang X, Marona-Lewicka D, Pfaff RC, Nichols DE (March 1994). "Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives". Pharmacology, Biochemistry, and Behavior 47 (3): 667–73. PMID 8208787.
- ^ Hofmann A. Psychotomimetic Drugs: Chemical and Pharmacological Aspects. Acta. Physiol. Pharmacol. Neerlandica. 1959;8:240-258.
- ^ US patent 2997470, Richard P. Pioch, "LYSERGIC ACID AMIDES", published 1956-03-05, issued 1961-08-22
- ^ David E. Nichols. LSD and Its Lysergamide Cousins. The Heffter Review of Psychedelic Research. 2001;2:80-87.
| v · d · eHallucinogens |
|
Psychedelics
5-HT2AR agonists |
Lysergamides: AL-LAD • ALD-52 • BU-LAD • CYP-LAD • DAM-57 • Diallyllysergamide • Ergometrine (Ergonovine, Ergobasine) • ETH-LAD • LAE-32 • LSA (Ergine, Lysergamide) • LSD • LSH • LPD-824 • LSM-775 • Lysergic Acid 2-Butyl Amide • Lysergic Acid 2,4-Dimethylazetidide • Lysergic Acid 3-Pentyl Amide • Methylergometrine • Methylisopropyllysergamide • Methysergide • MLD-41 • PARGY-LAD • PRO-LAD;
Phenethylamines: Aleph • 2C-B • 2C-B-Dragonfly · 2C-B-FLY • 2C-C-FLY • 2C-D-FLY • 2C-E-FLY • 2C-I-FLY • 2CBFly-NBOMe • 2C-T-7-FLY • 2C-C • 2C-C-NBOMe • 2C-CN-NBOMe • 2C-D • 2CD-5EtO • 2C-D-NBOMe • 2C-E • 2C-EF • 2C-E-NBOMe • 2C-F • 2C-F-NBOMe • 2C-G • 2C-G-NBOMe • 2C-H-NBOMe • 2C-I • 2C-N • 2C-N-NBOMe • 2C-O • 2C-O-4 • 2C-P • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-4-NBOMe • 2C-T-7 • 2C-T-7-NBOH • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-TFM-NBOMe • 2C-YN • 2CBCB-NBOMe • 25B-NBOMe • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 3C-E • 3C-P • 5-APB • 5-APDB • 6-APB • 6-APDB • Br-DFLY • DESOXY • DMMDA • DMMDA-2 • DOB • DOB-FLY • DOM-FLY • DOC • DOEF • DOET • DOF • DOI • DOM • DON • DOPR • DOTFM • Escaline • Ganesha • HOT-2 • HOT-7 • HOT-17 • IAP • Isoproscaline • Jimscaline • Lophophine • MDA • MDEA • MDMA • MMA • MMDA • MMDA-2 • MMDA-3a • MMDMA • Macromerine • Mescaline • Methallylescaline • NBOMe-mescaline • Proscaline • TCB-2 • TFMFly • TMA;
Piperazines: pFPP • TMFPP;
Tryptamines: 1-Methyl-5-methoxy-diisopropyltryptamine • 2,N,N-TMT • 4-HO-5-MeO-DMT • 4-Acetoxy-DET • 4-Acetoxy-DIPT • 4-Acetoxy-DMT • 4-Acetoxy-DPT • 4-Acetoxy-MiPT • 4-HO-DPT • 4-HO-MET • 4-Propionyloxy-DMT • 4-HO-MPMI • 5-Me-MIPT • 5-N,N-TMT • 5-AcO-DMT • 5-MeO-2,N,N-TMT • 5-MeO-α,N,N-TMT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DALT • 5-MeO-DET • 5-MeO-DIPT • 5-MeO-DMT • 5-MeO-DPT • 5-MeO-EiPT • 5-MeO-MET • 5-MeO-MIPT • 5-MeO-MPMI • 7,N,N-TMT • α,N,N-TMT • α-ET • α-MT • AL-37350A • Baeocystin • Bufotenin • DALT • DBT • DCPT • DET • DIPT • DMT • DPT • EiPT • Ethocin • Ethocybin • Iprocin • MET • Miprocin • MIPT • Norbaeocystin • PiPT • Psilocin • Psilocybin;
Others: AL-38022A • Ibogaine • Noribogaine • Voacangine
|
|
Dissociatives
NMDAR antagonists |
|
|
Deliriants
mAChR antagonists |
|
|
| Miscellaneous |
|
|
Wikimedia Foundation.
2010.
Look at other dictionaries:
Lysergic acid diethylamide — LSD redirects here. For other uses, see LSD (disambiguation). LSD 25 redirects here. For the dock landing ship, see USS San Marcos (LSD 25). For the Fringe episode, see Lysergic Acid Diethylamide (Fringe). Lysergic acid diethylamide … Wikipedia
Lysergamides — Amides of lysergic acid are collectively known as lysergamides.[1][2][3] General structure of Lysergamides Lysergamides, tabulated by structure Name R1 R6 … Wikipedia
ALD-52 — Systematic (IUPAC) name (6aR,9R) 4 acetyl N,N diethyl 7 methyl 4,6,6a,7,8,9 hexahydroindolo[4,3 fg]quinoline 9 carboxamide Clinical data … Wikipedia
Lysergic acid hydroxyethylamide — Systematic (IUPAC) name 9,10 didehydro N (1 hydroxyethyl) 6 methylergoline 8 carboxamide Clinical data … Wikipedia
Ayahuasca — This entry focuses on the Ayahuasca brew; for information on the vine of the same name, see Banisteriopsis caapi Ayahuasca cooking in the Napo region of Ecuador Ayahuasca (ayawaska pronounced [ajaˈwaska] in the Quechua language) is any of various … Wikipedia
Dimethyltryptamine — Systematic (IUPAC) name 2 (1H indol 3 yl) N … Wikipedia
Datura — This article is about the plant genus. For the Italian dance group, see Datura (band). For the former town in California, see Datura, California. For The Tori Amos song, see Datura (song). Datura Datura stramonium … Wikipedia
Mandrake (plant) — Mandrake root redirects here. For the Deep Purple song, see Mandrake Root. Mandragora redirects here. For other uses, see Mandragora (disambiguation). Mandrake Scientific classification Kingdom … Wikipedia
Phencyclidine — Systematic (IUPAC) name … Wikipedia
Solanaceae — Nightshade redirects here. For other uses, see Nightshade (disambiguation). Solanaceae A flowering Brugmansia suaveolens from the US Botanic Garden Scientific classification … Wikipedia
 (what is this?) (verify)
(what is this?) (verify)