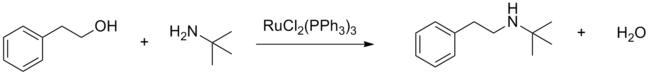- Dichlorotris(triphenylphosphine)ruthenium(II)
-
Dichlorotris(triphenylphosphine)ruthenium(II) 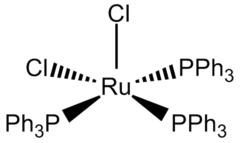 Dichlorotris(triphenylphosphine)ruthenium(II)Other namesRuthenium tris(triphenylphosphine) dichloride; Tris(triphenylphosphine)dichlororuthenium; Tris(triphenylphosphine)ruthenium dichloride;Tris(triphenylphosphine)ruthenium(II) dichloride
Dichlorotris(triphenylphosphine)ruthenium(II)Other namesRuthenium tris(triphenylphosphine) dichloride; Tris(triphenylphosphine)dichlororuthenium; Tris(triphenylphosphine)ruthenium dichloride;Tris(triphenylphosphine)ruthenium(II) dichlorideIdentifiers CAS number 15529-49-4 
ChemSpider 76650 
EC number 239-569-7 Jmol-3D images Image 1 - [Ru+2].[Cl-].[Cl-].c3c(P(c1ccccc1)c2ccccc2)cccc3.c1ccccc1P(c2ccccc2)c3ccccc3.c1ccccc1P(c2ccccc2)c3ccccc3
Properties Molecular formula C54H45Cl2P3Ru Molar mass 958.83 g/mol Appearance Black Crystals or Red-Brown Density 1.43 g/cm3 Melting point 133 °C, 406 K, 271 °F
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. This chocolate brown solid is a precursor to other complexes including those used as homogeneous catalysis.
Contents
Synthesis and basic properties
RuCl2(PPh3)3 is the product of the reaction of ruthenium trichloride trihydrate with a methanolic solution of triphenylphosphine.[1][2]
- 2 RuCl3(H2O)3 + 7 PPh3 → 2 RuCl2(PPh3)3 + 2 HCl + 5 H2O + 1 OPPh3
When conducted in the presence of larger excess of triphenylphosphine, the synthesis affords black RuCl2(PPh3)4.
The coordination sphere of RuCl2(PPh3)3 can be viewed as either five-coordinate or octahedral. One coordination site is occupied by one of the hydrogen atoms of a phenyl group.[3] This Ru---H agostic interaction is long (2.59 Å) and weak. The low symmetry of the compound is reflected by the differing lengths of the Ru-P bonds: 2.374, 2.412, and 2.230 Å.[4] The Ru-Cl bond lengths are both 2.387 Å.
Substitution reactions
The triphenylphosphine ligands are labile and are readily substituted by other ligands. Dichlorotris(triphenylphosphine)ruthenium(II) reacts with carbon monoxide to produce the all trans isomer of dichloro(dicarbonyl)bis(triphenylphosphine)ruthenium(II).
- RuCl2(PPh3)3 + 2 CO → trans,trans,trans-RuCl2(CO)2(PPh3)2 + PPh3
This kinetic product isomerizes to the cis adduct during recrystallization. trans-RuCl2(dppe)2 forms upon treating RuCl2(PPh3)3 with dppe.
- RuCl2(PPh3)3 + 2 dppe → RuCl2(dppe)2 + 3 PPh3
Use in organic synthesis
RuCl2(PPh3)3 facilitates oxidations, reductions, cross-couplings, cyclizations, and isomerization. It is used in the Kharasch addition of chlorocarbons to alkenes.[5]
Dichlorotris(triphenylphosphine)ruthenium(II) serves as a precatalyst for the hydrogenation of alkenes, nitro compounds, ketones, carboxylic acids, and imines. On the other hand, it catalyzes the oxidation of alkanes to tertiary alcohols, amides to t-butyldioxyamides, and tertiary amines to α-(t-butyldioxyamides) using tert-butyl hydroperoxide. Using other peroxides, oxygen, and acetone, the catalyst can oxidize alcohols to aldehydes or ketones. Using dichlorotris(triphenylphosphine)ruthenium(II) the N-alkylation of amines with alcohols is also possible (see "borrowing hydrogen").[5]
RuCl2(PPh3)3 efficiently catalyzes carbon-carbon bond formation from cross couplings of alcohols through C-H activation of sp3 carbons in the presence of a Lewis acid.[6]
RuCl2(PPh3)3 efficiently catalyzes the decomposition of formic acid into carbon dioxide and hydrogen gas in the presence of an amine.[7] Since carbon dioxide can be trapped and hydrogenated on an industrial scale, formic acid represents a potential storage and transportation medium.
References
- ^ Stephenson, T. A.; Wilkinson, G. “New Complexes of Ruthenium (II) and (III) with Triphenylphosphine, Triphenylarsine, Trichlorostannate, Pyridine, and other Ligands”, J. Inorg. Nucl. Chem., 1966, 28, 945-956. doi:10.1016/0022-1902(66)80191-4
- ^ P. S. Hallman, T. A. Stephenson, G. Wilkinson "Tetrakis(Triphenylphosphine)Dichloro-Ruthenium(II) and Tris(Triphenylphosphine)-Dichlororuthenium(II)" Inorganic Syntheses, 1970 Volume 12, . doi:0.1002/9780470132432.ch40
- ^ Sabo-Etienne, S.; Gellier, M., “Ruthenium: Inorganic and Coordination Chemistry”, Encyclopedia of Inorganic Chemistry, 2006, John Wiley & Sons. doi:10.1002/0470862106.ia208
- ^ La Placa, S. J.; Ibers, J.A., “A Five-Coordinated d6 Complex: Structure of Dichlorotris(triphenylphosphine)ruthenium(II)”, Inorganic Chemistry, 1965, 4, 778-78. doi:10.1021/ic50028a002
- ^ a b Plummer, J. S.; Shun-Ichi, M.; Changjia, Z. “Dichlorotris(triphenylphosphine)ruthenium(II)”, e-EROS Encyclopedia of Reagents for Organic Synthesis, 2010, John Wiley. doi:10.1002/047084289X.rd137.pub2
- ^ Shu-Yu, Z.; Yong-Qiang, T.; Chun-An, F.; Yi-Jun, J.; Lei, S.; Ke, C.; En, Z.; “Cross-Coupling Reactions between alcohols through sp3 C-H Activation Catalyzed by a Ruthenium/Lewis Acid System” Chem. Eur. J., 2008, 14, 10201-10205. doi:10.1002/chem.200801317
- ^ Loges, B.; Boddien, A.; Junge, H.; Beller, M., “Controlled Generation of Hydrogen from Formic Acid Amine Adducs at Room Temperature and Application in H2/O2 Fuel Cells”, Angew. Chem. Int. Ed., 2008, 47, 3962-3965. doi: 10.1002/anie.200705972
Ruthenium compounds RuB2 · RuO2 · RuCl3 · RuO4 · N(C3H7)4RuO4 · C72H42N6Na4O18RuS6 · Ru3(CO)12 · (Ru(bipy)3)Cl2 · C62H42O6Ru2 · C54H45Cl2P3Ru · C8H24Cl2O4RuS4 · C56H45O2P3Ru · · C20H28Cl4Ru2 · C41H35ClP2Ru · (C5H5)2Ru
Categories:- Ruthenium compounds
Wikimedia Foundation. 2010.