- Octasulfur
-
Octasulfur 

 Systematic nameOctathiocane[1]Other namesOctacyclosulfur[citation needed]
Systematic nameOctathiocane[1]Other namesOctacyclosulfur[citation needed]Identifiers CAS number 10544-50-0 PubChem 66348 ChemSpider 59726 
MeSH Cyclooctasulfur ChEBI CHEBI:29385 ChEMBL CHEMBL1235452 
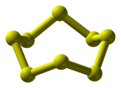
Gmelin Reference 2973 Jmol-3D images Image 1 - S1SSSSSSS1
Properties Molecular formula S8 Molar mass 256.52 g mol−1 Exact mass 255.776565520 g mol-1 Appearance Vivid, yellow, translucent crystals Density 2.07 g cm-3 Melting point 119 °C, 392 K, 246 °F
Boiling point 159 °C, 432 K, 318 °F (decomposes)
log P 6.117 Thermochemistry Specific heat capacity, C 22.75 J K-1 mol-1 Related compounds Related compounds Hexathiane Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Octasulfur is a cyclosulfane with the molecular formula S8. It is a simple yellow coloured sulfur. It is also the final member of the thiocane heterocylic series, where every carbon is substituted with a sulfur atom.
Octasulfur exists as three distinct polymorphs, rhombohedral, and two monoclinic forms, of these only two are stable at standard conditions. The rhombohedral crystal form is the accepted standard. The remaining polymorph is only stable between 96 °C and 115 °C at 100 kPa, above 115 octasulfur starts to slowly disproportionate. However, if heated fast enough, with minimal degradation, octasulfur will melt at 119 °C, before being completely degraded above 159 °C.
Octasulfur forms several sulfur allotropes:
α-Sulfur
β-Sulfur
γ-Sulfur
λ-Sulfurλ-Sulfur is the liquid form of octasulfur, from which γ-sulfur can be crystallised by quenching. If λ-sulfur is crystallised slowly, it will revert back to β-sulfur. Since it must have been heated over 115 °C, neither crystallised β-sulfur, or γ-sulfur will be pure. The only known method of obtaining pure γ-sulfur, is by crystallising from solution.
Octasulfur easily forms large sized crystals, these crystals are typically vivid yellow in colour, and are somewhat translucent. As is typical of other crystalline compounds, pulverised sulfur is completely different in appearance - it is a paler colour, and opaque as is shown in the image.
References
- ^ "cyclooctasulfur (CHEBI:29385)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. IUPAC Name. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=29385.
Periodic table H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo Alkali metals Alkaline earth metals Lanthanides Actinides Transition metals Other metals Metalloids Other nonmetals Halogens Noble gases Unknown chem. properties Large version Categories:- Agricultural chemicals
- Biology and pharmacology of chemical elements
- Dietary minerals
- Native element minerals
- Inorganic polymers
- Nonmetals
- Sulfur
Wikimedia Foundation. 2010.
