- (Mesitylene)molybdenum tricarbonyl
-
(Mesitylene)molybdenum tricarbonyl 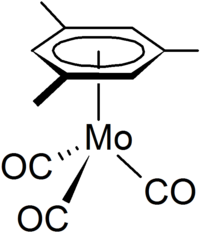 (1,3,5-Trimethylbenzene) molybdenum tricarbonyl)Other namesMolybdenum, tricarbonyl[(1,2,3,4,5,6-η)-1,3,5-trimethylbenzene]- (9CI) Mesitylenemolybdenum tricarbonyl (6CI); Molybdenum, tricarbonyl(mesitylene)- (7CI,8CI); Benzene, 1,3,5-trimethyl-, molybdenum complex; (1,3,5-Trimethylbenzene)molybdenum tricarbonyl; Mesitylenetricarbonylmolybdenum; Tricarbonyl(1,3,5-trimethylbenzene)molybdenum; Tricarbonyl(mesitylene)molybdenum; Tricarbonyl(η-mesitylene)molybdenum; Tricarbonyl(η6-1,3,5-trimethylbenzene)molybdenum; Tricarbonyl(η6-mesitylene)molybdenum
(1,3,5-Trimethylbenzene) molybdenum tricarbonyl)Other namesMolybdenum, tricarbonyl[(1,2,3,4,5,6-η)-1,3,5-trimethylbenzene]- (9CI) Mesitylenemolybdenum tricarbonyl (6CI); Molybdenum, tricarbonyl(mesitylene)- (7CI,8CI); Benzene, 1,3,5-trimethyl-, molybdenum complex; (1,3,5-Trimethylbenzene)molybdenum tricarbonyl; Mesitylenetricarbonylmolybdenum; Tricarbonyl(1,3,5-trimethylbenzene)molybdenum; Tricarbonyl(mesitylene)molybdenum; Tricarbonyl(η-mesitylene)molybdenum; Tricarbonyl(η6-1,3,5-trimethylbenzene)molybdenum; Tricarbonyl(η6-mesitylene)molybdenumIdentifiers CAS number 12089-15-5 Properties Molecular formula C12H12MoO3 Molar mass 300.16 g mol−1 Density 1.455 g/cm3  tricarbonyl (verify) (what is:
tricarbonyl (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references (Mesitylene)molybdenum tricarbonyl is an organomolybdenum compound derived from the aromatic compound mesitylene (1,3,5-trimethylbenzene) and molybdenum carbonyl. It exists as a pale yellow crystals, which are soluble in organic solvents but decompose quickly when in solution. It has been examined as a catalyst and reagent.
Contents
Synthesis
(Mesitylene)molybdenum tricarbonyl arises from the reaction of molybdenum hexacarbonyl with hot mesitylene:[1]
- Mo(CO)6 + (CH3)3C6H3 → Mo(CO)3[(CH3)3C6H3] + 3 CO
It can also be synthesized, with good yields by displacement of pyridine ligands of the trispyridine complex Mo(CO)3(pyridine)3 in the presence of Lewis acids. This reaction proceeds at lower temperatures of the compound than the direct method
- Py3Mo(CO)3 + (CH3)3C6H3 + 3BF3·O(C2H5)2 → [(CH3)3C6H3]Mo(CO)3 + 3PyBF3
Structure and properties
The mesitylene group is bonded to the molybdenum centre through delocalized π - electron ring. The aromaticity of the ligand is indicated by its ability to undergo Friedel-Crafts reactions, e.g. with acetyl chloride. Such reactions are slower on the tricarbonyl(mesitylene)molybdenum than benzene, which suggests that the electron density contributes to the bonding to the molybdenum.
The tricarbonyl(mesitylene)molybdenum complex adopts a near C3v symmetry with the three carbonyl groups occupying an eclipsed arrangement relative to the three methyl groups. The mesityl group is η6 to the molybdenum central metal atom, which lies 0.009 Å away from the ring centre and the methyl groups on the benzene are bent out of plane by 0.035 Å due to steric interaction with the carbonyl groups.[2][3]
Reactions
The arene can be displaced by the trimethylphosphite via a Sn2 type mechanism to give the fac-tricarbonyltris(trimethyl phosphite)molybdenum.[4]
- (CH3O)3P + [(CH3)3C6H3]Mo(CO)3 → fac-[(CH3)3C6H3]Mo(CO)3 + (CH3)3C6H3
The tricarbonyl(mesitylene)molybdenum complex can be used an electron donor,.[4][5]
Tricarbonyl(mesitylene)molybdenum can act as a catalyst for the polymerisation of phenylacetylene.[6] The Molybdenum complex is activated with an oxidant such as chloranil. The result of the charge transfer facilitates ring slippage and the mesitylene group changes from η6 to η2 this allows the phenylacetylene monomer units to bind to the metal centre.
References
- ^ G.S. Girolami,T.B. Rauchfuss, R.J. Angelici (1999). Synthesis and Techniques in Inorganic Chemistry.
- ^ D.E. Koshland, S.E. Myers, (1973). "The Crystal Structures of 1,3,5-Trimethylbenzene tricarbonylmolybdenum and hexamethylbenzene tricarbonylmolybdenum". Acta Cryst. 4 (7): 836–866. doi:10.1107/S056774087700764X.
- ^ O.T. Beachley, T.L. Royster, J. Youngs, A. Eugene (1989). "Chemistry of mesitylgallium(III) derivatives as arene ligands in metal carbonyl complexes". Organometallics 8: 1679–88. doi:10.1021/om00109a017.
- ^ a b M. Tamm, R.J. Baker (2007). "Molybdenum Compounds with CO or Isocyanoides". IComprehensive Organometallic Chemistry III from Fundamentals to Applications 5: 319–512. doi:10.1016/B0-08-045047-4/00071-6.
- ^ A. Pidlock, J.D. Smith, B.W. Taylor (1967). "Ligand Displacement Reactions. Part I. Kinetics of the Reaction Between Trimethyl Phosphite and some Tricarbonyl(arene) Molybdenum Complexes". J. Chem. Soc: 872–876.
- ^ M.B. Mula, A.J. Beaumont, K.O. Doyle, M.L. Gallagher, A.D. Rooney (1999). "Charge-trnasfer complexes of arene-molybdenum-tricarbonyl complexes as heterogenous metathesis catalysts for the polymerisation of phenylacetylene". Journal of Molecular Catalysis 148: 23–28. doi:10.1016/S1381-1169(99)00040-0.
Categories:- Organomolybdenum compounds
Wikimedia Foundation. 2010.
