- Bromoform
-
Bromoform 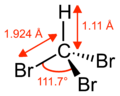
 TribromomethaneOther namesBromoform, Methyl tribromide, Methenyl tribromide, R-20B3, UN 2515
TribromomethaneOther namesBromoform, Methyl tribromide, Methenyl tribromide, R-20B3, UN 2515Identifiers CAS number 75-25-2 
PubChem 5558 ChemSpider 13838404 
UNII TUT9J99IMU 
EC number 200-854-6 DrugBank DB03054 KEGG C14707 
ChEBI CHEBI:38682 
ChEMBL CHEMBL345248 
RTECS number PB5600000 Jmol-3D images Image 1 - BrC(Br)Br
Properties Molecular formula CHBr3 Molar mass 252.73 g mol−1 Appearance Colorless to yellow liquid with a sweet odor Density 2.889 g/cm3 at 15 °C Melting point 8.0 °C
Boiling point 149.1 °C
Solubility in water 3.2 g/l at 30 °C log P 2.38 Vapor pressure 660 Pa at 20 °C Hazards MSDS External MSDS EU classification Toxic (T), Dangerous for the environment (N), Carc. Cat. 3 R-phrases R23, R36, R38, R51, R53 S-phrases S28, S45, S61 NFPA 704 U.S. Permissible
exposure limit (PEL)0.5 ppm Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Bromoform (CHBr3) is a pale yellowish liquid with a sweet odor similar to chloroform, a halomethane or haloform. Its refractive index is 1.595 (20°C, D). Bromoform is produced naturally by phytoplankton and seaweeds in the ocean and this is thought to be the predominant source to the environment.[1] However, locally significant amounts of bromoform enter the environment formed as disinfection byproducts known as the trihalomethanes when chlorine is added to drinking water to kill bacteria. It is somewhat soluble in water and readily evaporates into the air. Bromoform is a confirmed animal carcinogen; (ACGIH 2004). Carcinogen category: 3B; (DFG 2004).
Bromoform is one of the trihalomethane closely related with fluoroform, chloroform, and iodoform. It is the main trihalomethane produced in salt swimming pools with some public swimming pools found to contain up to 1.3PPM bromoform.[2] Occupational skin exposure limits are set at 0.1PPM.
Bromoform can be absorbed into the body by inhalation and through the skin. The substance is irritating to the respiratory tract, the eyes, and the skin, and may cause effects on the central nervous system and liver, resulting in impaired functions. It is soluble in about 800 parts water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone, and oils. Its LD50 is 7.2 mmol/kg in mice, or 1.8g/kg.
The substance may be hazardous to the environment and special attention should be given to aquatic organisms. It is strongly advised not to let the chemical enter into the environment because it persists in the environment.
It can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminum bromide.
Uses
Only small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, sedative and flame retardant, but now it is mainly used as a laboratory reagent.
Due to bromoform's relatively high density, it is commonly used for the separation of minerals. In one application of the technique, two samples can be separated by bromoform in a test tube or equivalent glassware. The top layer, which contains the lighter minerals, can be removed from the bottom layer, which contains the heavier minerals.
This ability of bromoform to support the weight of some solids is explained by the laws of buoyancy. A solid will float in a liquid if its density is less than that of the liquid. Likewise, a solid will sink if its density is more than that of the liquid. If a liquid is to be used to separate minerals according to their densities, it should have a density that is in between that of the minerals. Bromoform has a high density compared to that of other liquids, and so it is ideal for this application. If the density of bromoform is slightly too high, then it can be brought down by mixing it with a small amount of fully miscible liquid of lesser density.
References
- Betterton E. A., Arnold R. G., Kuhler R. J., Santo G. A. (June 2005). "Reductive dehalogenation of bromoform in aqueous solution". Environ Health Perspect. (Brogan &) 103: 89–91(3). doi:10.2307/3432487. JSTOR 3432487. PMC 1519304. PMID 8565919. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1519304. Retrieved 2007-07-03. PDF
- U.S. Department of Health and Human Services. Toxicological Profile for Bromoform and Dibromochloromethane [1]. August 2005.
- ^ Palmer C J and Reason C J (2009), Relationships of surface bromoform concentrations with mixed layer depth and salinity in the tropical oceans (2009), Global Biogeochemical Cycles, 23, GB2014.
- ^ Beech AJ et al (1980) Nitrates, Chlorates and Trihalomethanes in Swimming Pool Water. Am J Public Health, 70(1), 79-82
External links
- International Chemical Safety Card 0108
- NIOSH Pocket Guide to Chemical Hazards 0066
- MSDS at Oxford University
- Entry at chemicalland21.com
- Toxicological profile for bromoform and dibromochlormethane
- Toxicity summary
- IARC Summaries & Evaluations: Vol. 62 (1991), Vol. 71 (1999)
- ChemSub Online: Bromoform
Halomethanes Monosubstituted Disubstituted Trisubstituted Categories:- Organobromides
- Halomethanes
- Halogenated solvents
- Flame retardants
- IARC Group 3 carcinogens
Wikimedia Foundation. 2010.


