- Synechococcus
Taxobox | name = "Synechococcus"
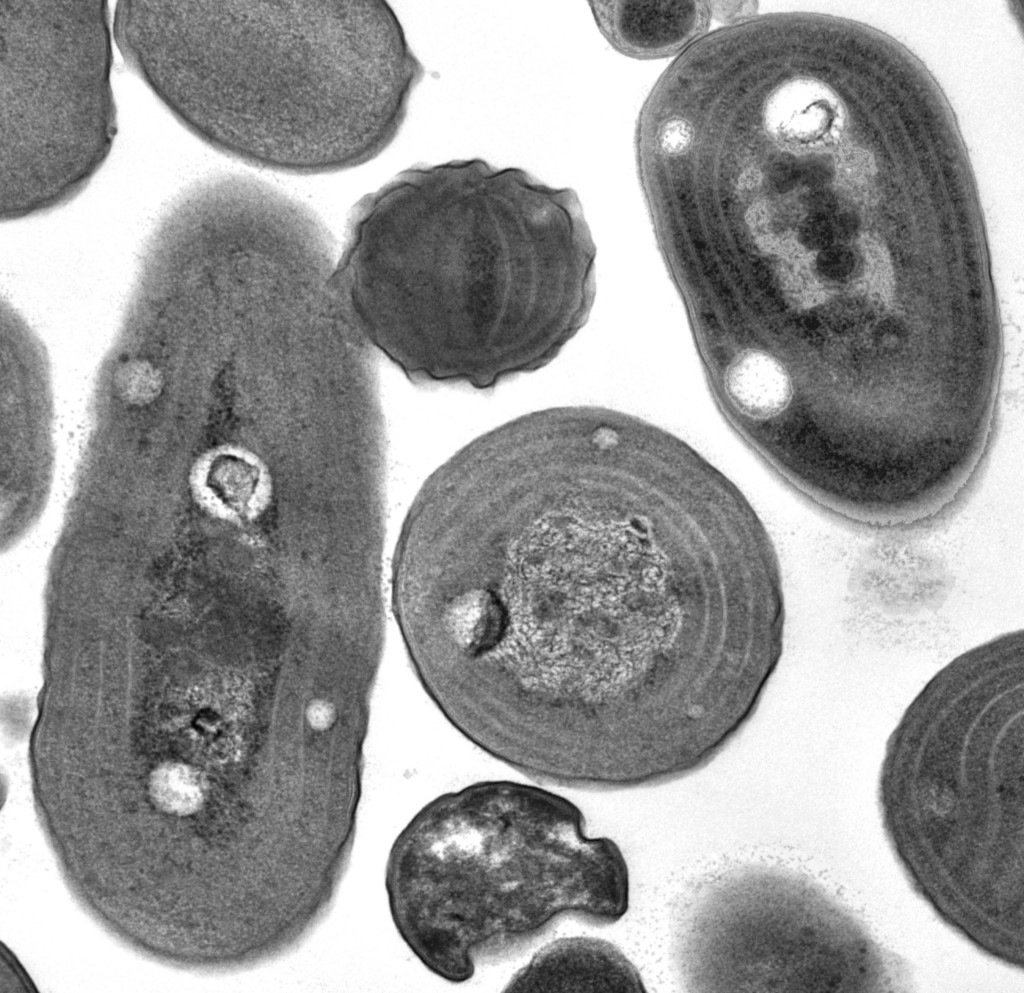
image_width = 200px
image_caption = TEM of "Synechococcus"
regnum =Bacteria
divisio =Cyanobacteria
ordo =Synechococcales
familia =Synechococcaceae
genus = "Synechococcus"
genus_authority = Nägeli, 1849
subdivision_ranks = Species
subdivision = "See text""Synechococcus" is a unicellular
cyanobacterium that is very widespread in the marine environment. Its size varies from 0.8 µm to 1.5 µm. Thephotosynthetic coccoid cells are preferentially found in well–litsurface waters where it can be very abundant (generally 1,000 to 200,000 cells permillilitre ). Many freshwater species of "Synechococcus" have also been described.The
genome of "Synechococcus elongatus" strain PCC7002 has a size of 2.7 Mbp, that of the oceanic strain WH8102 is 2.4 Mbp.Introduction
"Synechococcus" is one of the most important components of the prokaryotic
autotroph icpicoplankton in thetemperate to tropical oceans. The genus was first described in 1979 [cite journal |quotes=no |author=P. W. Johnson & J. M. Sieburth |year=1979 |title=Chroococcoid cyanobacteria in the sea: a ubiquitous and diverse phototrophic biomass |journal=Limnology and Oceanography |volume=24 |pages=928–935] cite journal |quotes=no |author=J. B. Waterbury, S. W. Watson, R. R. L. Guillard & L. E. Brand |year=1979 |title=Wide-spread occurrence of a unicellular, marine planktonic, cyanobacterium |journal=Nature |volume=277 |pages=293–294 |url=http://www.nature.com/nature/journal/v277/n5694/abs/277293a0.html |doi=10.1038/277293a0 |format=abstract page] , and was originally defined to include "small unicellular cyanobacteria with ovoid to cylindrical cells that reproduce by binary traverse fission in a single plane and lack sheaths" cite journal |quotes=no |author=R. Rippka, J. Deruelles, J. B. Waterbury, M. Herdman & R. Y. Stanier |year=1979 |title=Generic assignments, strains histories and properties of pure cultures of cyanobacteria |journal=Society for General Microbiology |volume=111 |pages=1–61] . This definition of the genus "Synechococcus" contained organisms of considerable genetic diversity and was later subdivided into subgroups based on the presence of the accessory pigmentphycoerythrin . The marine forms of "Synechococcus" are coccoid cells between 0.6 µm and 1.6 µm in size. They aregram negative cells with highly structured cell walls that may contain projections on their surface [cite journal |quotes=no |author=F. O. Perkins, L. W. Haas, D. E. Phillips & K. L. Webb |year=1981 |title=Ultrastructure of a marine "Synechococcus" possessing spinae |journal=Canadian Journal of Microbiology |volume=27 |pages=318–329] . Electron microscopy frequently reveals the presence of phosphate inclusions,glycogen granules and more importantly highly structuredcarboxysome s.Cells are known to be
motile by a gliding type method [cite book |author=R. W. Castenholz |year=1982 |chapter=Motility and taxes |editor=N. G. Carr & B. A. Whitton |title=The biology of cyanobacteria |publisher=University of California Press, Berkeley and Los Angeles |pages=413–439 |id=ISBN 0-520-04717-6] and a novel uncharacterized, non-phototactic swimming method [cite journal |quotes=no |author=J. B. Waterbury, J. M. Willey, D. G. Franks, F. W. Valois & S. W. Watson |year=1985 |title=A cyanobacterium capable of swimming motility |journal=Science |volume=30 |pages=74–76 |doi=10.1126/science.230.4721.74 |url=http://www.sciencemag.org/cgi/content/abstract/230/4721/74 |pmid=17817167 |format=abstract page] that does not involve flagellar motion. While some cyanobacteria are capable of photoheterotrophic or even chemoheterotrophic growth, all marine "Synechococcus" strains appear to be obligate photoautotrophs cite journal |quotes=no |author=J. B. Waterbury, S. W. Watson, F. W. Valois & D. G. Franks |year=1986b |title=Biological and ecological characterization of the marine unicellular cyanobacterium "Synechococcus" |journal=Canadian Bulletin of Fisheries and Aquatic Sciences |volume=214 |pages=71–120] that are capable of supporting their nitrogen requirements using nitrate, ammonia or in some casesurea as a solenitrogen source. Marine "Synechococcus" are traditionally not thought to fix nitrogen (This perception may be changing).Pigments
The main photosynthetic pigment in "Synechococcus" in
chlorophyll a , while its major accessory pigments arephycobilliprotein s . The four commonly recognizedphycobillin s arephycocyanin ,allophycocyanin ,allophycocyanin B andphytoerythrin [cite journal |quotes=no |author=R. Y. Stanier & G. Cohen-Bazire |year=1977 |title=Phototrophic prokaryotes: the cyanobacteria |journal=Annual Review of Microbiology |volume=31 |pages=255–274 |doi=10.1146/annurev.mi.31.100177.001301] . In addition "Synechococcus" also containszeaxanthin but no diagnostic pigment for this organism is known. Zeaxanthin is also found in "Prochlorococcus ",red algae and as a minor pigment in some chlorophytes andeustigmatophyte s. Similarly phycoerythrin is also found in rhodophytes and somecryptomonad s .Phylogeny
Phylogenetic description of "Synechococcus" is difficult. Isolates are morphologically very similar, yet exhibit a G+C content ranging from 39% to 71% , illustrating the large genetic diversity of this provisional taxon. Initially attempts were made to divide the group into three sub-clusters, each with a specific range of genomic G+C content [cite journal |quotes=no |author=R. Rippka & G. Cohen-Bazire |year=1983 |title=The Cyanobacteriales: a legitimate order based on type strains "Cyanobacterium stanieri"? |journal=
Annals of Microbiology |volume=134B |pages=21–36] . The observation that open-ocean isolates alone nearly span the complete G+C spectrum however indicates that "Synechococcus" is composed of at least several species. Bergey's Manual (Herdman "et al." 2001) now divides "Synechococcus" into five clusters (equivalent to genera) based on morphology, physiology and genetic traits.Cluster one includes relatively large (1–1.5 µm) non-motile obligate photoautotrophs that exhibit low salt tolerance. Reference strains for this cluster are PCC6301 (formerly "Anacycstis nidulans") and PCC6312, which were isolated from freshwater in
Texas andCalifornia respectively . Cluster 2 also is characterized by low salt tolerance. Cells are obligate photoautrotrophs, lack phycoerythrin and are thermophilic. The reference strain PCC6715 was isolated from a hot spring inYellowstone National Park [cite journal |quotes=no |author=D. L. Dyer & R. D. Gafford |year=1961 |title=Some characteristics of a thermophilic blue-green alga |journal=Science |volume=134 |pages=616–617 |doi=10.1126/science.134.3479.616 |pmid=13725365] . Cluster 3 includes phycoerythrin lacking marine "Synechococcus" that are euryhaline i.e. capable of growth in both marine and fresh water environments. Several strains, including the reference strain PCC7003 are facultative heterotrophs and require vitamin B12 for growth. Cluster 4 contains a single isolate, PCC7335. This strain is obligate marine [cite book |author=J. B. Waterbury & R. Y. Stanier |year=1981 |chapter=Isolation and growth of cyanobacteria from marine and hypersaline environments |editor=Starr, Stulp, Truper, Balows, Schleeper |title=The prokaryotes: a handbook on habitats, isolation, and identification of bacteria, Vol 1 |publisher=Springer-Verlag , Berlin |pages=221–223 |id=ISBN 0-38-708871-7] . This strain contains phycoerthrin and was first isolated from the intertidal zone inPuerto Peñasco ,Mexico . The last cluster contains what had previously been referred to as ‘marine A and B clusters’ of "Synechococcus". These cells are truly marine and have been isolated from both the coastal and the open ocean. All strains are obligate photoautrophs and are around 0.6–1.7 µm in diameter. This cluster is however further divided into a population that either contains (cluster 5.1) or does not contain (cluster 5.2) phycoerythrin. The reference strains are WH8103 for the phycoerythrin containing strains and WH5701 for those strains that lack this pigment (Waterbury "et al." 1986b). More recently Badger proposed the division of the cyanobacteria into a α- and a β-subcluster based on the type of rbcL (large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase) found in these organisms [cite journal |quotes=no |author=M. R. Badger, D. Hanson & G. D. Price |year=2002 |title=Evolution and diversity of CO2 concentrating mechanism in cyanobacteria |journal=Functional Plant Biology |volume=29 |pages=161–175 |url=http://jxb.oxfordjournals.org/cgi/reprint/54/383/609 |doi=10.1071/PP01213] . α-cyanobacteria were defined to contain a form IA, while β-cyanobacteria were defined to contain a form IB of this gene. In support for this division Badger analyzes the phylogeny of carboxysomal proteins, which appear to support this division. Also, two particular bicarbonate transport systems appear to only be found in α-cyanobacteria, which lack carboxysomal carbonic anhydrases.Ecology and distribution
"Synechococcus" has been observed to occur at concentrations ranging between a few cells per ml to 106 cells per ml in virtually all regions of the oceanic
euphotic zone except in samples from theMcMurdo Sound andRoss Ice Shelf inAntarctica . Cells are generally much more abundant in nutrient rich environments than in the oligotrophic ocean and prefer the upper well lit portion of the euphotic zone cite journal |quotes=no |author=F. Partensky, J. Blanchot & D. Vaulot |year=1999a |chapter=Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: a review |editor=Charpy L, Larkum AWD |title=Marine cyanobacteria. no. NS 19. Bulletin de l'Institut Oceanographique Monaco, Vol NS 19 |publisher=Musee oceanographique, Monaco |pages=457–475] . "Synechococcus" has also been observed to occur at high abundances in environments with low salinities and/or low temperatures. Synechococcus is usually far outnumbered by "Prochlorococcus" in all environments, where they co-occur. Exceptions to this rule are areas of permanently enriched nutrients such asupwelling areas andcoastal watershed s . In the nutrient deplete areas of the oceans, such as the central gyres, "Synechococcus" is apparently always present, although only at low concentrations ranging from a few to 4×10³ cells per ml cite journal |quotes=no |author=W. K. W. Li |year=1995 |title=Composition of ultraphytoplankton in the central North Atlantic |journal=Marine Ecology Progress Series |volume=122 |pages=1–8 |url=http://www.int-res.com/articles/meps/122/m122p001.pdf |doi=10.3354/meps122001] cite journal |quotes=no |author=R. J. Olson, S. W. Chisholm, E. R. Zettler & E. V. Armbrust |year=1990b |title=Pigment size and distribution of Synechococcus in the North Atlantic and Pacific oceans |journal=Limnology and Oceanography |volume=35 |pages=45–58] cite journal |quotes=no |author=J. Blanchot, M. Rodier & A. LeBouteiller |year=1992 |title=Effect of El Niño Southern Oscillation events on the distribution and abundance of phytoplankton in the Western Pacific Tropical Ocean along 165°E |journal=J. Plank. Res |volume=14 |pages=137–156 |url=http://plankt.oxfordjournals.org/cgi/content/abstract/14/1/137 |doi=10.1093/plankt/14.1.137 |format=abstract page] cite journal |quotes=no |author=L. Campbell & D. Vaulot |year=1993 |title=Photosynthetic picoplankton community structure in the stubtropical North Pacific Ocean new Hawaii (station ALOHA) |journal=Deep Sea Research I |volume=40 |pages=2043–2060 |doi=10.1016/0967-0637(93)90044-4] [cite journal |quotes=no |author=J. Blanchot & M. Rodier |year=1996 |title=Picophytoplankton abundance and biomass in the western tropical Pacific Ocean during the 1992 El Nino year: results from flow cytometry |journal=Deep-sea Research I |volume=43 |pages=877–895 |doi=10.1016/0967-0637(96)00026-X] . Vertically "Synechococcus" is usually relatively equitably distributed throughout the mixed layer and exhibits an affinity for the higher light regime. Below the mixed layer, cell concentrations rapidly decline. Vertical profiles are however strongly influenced by hydrologic conditions and can be very variable both seasonally and spatially. Overall "Synechococcus" abundance often parallels that of "Prochlorococcus" in the water column. In the Pacific HNLC (High Nutrient Low Chlorophyll) zone and in temperate open seas where stratification was recently established both profiles parallel each other and exhibit abundance maxima just about the SCM [cite journal |quotes=no |author=M. R. Landry, J. Kirshtein & J. Constantinou |year=1996 |title=Abundances and distributions of picoplankton populations in the central equatorial Pacific from 12°N to 12°S, 140°W |journal=Deep-Sea Research II |volume=43 |pages=871–890 |doi=10.1016/0967-0645(96)00018-5] .The factors controlling the abundance of "Synechococcus" still remain poorly understood, especially considering that even in the most nutrient deplete regions of the central gyres, where cell abundances are often very low, population growth rates are often high and not very drastically limited . Factors such as grazing, viral mortality, genetic variability, light adaptation, temperature as well as nutrients are certainly involved, but remain to be investigated on a rigorous and global scale. Despite the uncertainties it has been suggested that there is at least a relationship between ambient nitrogen concentrations and "Synechococcus" abundance and an inverse relationship to "Prochlorococcus" in the upper euphotic zone, where light is not limiting. One environment where "Synechococcus" thrives particularly well are coastal plumes of major rivers [cite journal |quotes=no |author=J. H. Paul, B. Wawrik & A. Alfreider |year=2000 |title=Micro- and macrodiversity in rbcL sequences in ambient phytoplankton populations from the southeastern Gulf of Mexico |journal=
Marine Ecology Progress Series |volume=198 |pages=9–18 |url=http://www.int-res.com/articles/meps/198/m198p009.pdf |doi=10.3354/meps198009] [cite journal |quotes=no |author=B. Wawrik, D. John, M. Gray, D. A. Bronk & J. H. Paul |year=2004 |title=Preferential uptake of ammonium in the presence of elevated nitrate concentrations by phytoplankton in the offshore Mississippi Plume |journal=Aquatic Microbial Ecology |volume=35 |pages=185–196] [cite journal |quotes=no |author=B. Wawrik & J. H. Paul |year=2004 |title=Phytoplankton community structure and productivity along the axis of the Mississippi Plume |journal=Aquatic Microbial Ecology |volume=35 |pages=175–184 |doi=10.3354/ame035185] [cite journal |quotes=no |author=B. Wawrik, J. H. Paul, L. Campbell, D. Griffin, L. Houchin, A. Fuentes-Ortega & F. Müller-Karger |year=2003 |title=Vertical structure of the phytoplankton community associated with a coastal plume in the Gulf of Mexico |journal=Marine Ecology Progress Series |volume=251 |pages=87–101 |url=http://www.int-res.com/articles/meps2003/251/m251p087.pdf |doi=10.3354/meps251087] . Such plumes are coastally enriched with nutriets such as nitrate and phosphate, which drives large phytoplankton blooms. High productivity in coastal river plumes is often associated with large populations of "Synechococcus" and elevated form IA (cyanobacterial) "rbcL" mRNA.It should also be noted that "Prochlorococcus" is thought to be at least 100 times more abundant than Synechococcus in warm oligotrophic waters . Assuming average cellular carbon concentrations it has thus been estimated that "Prochlorococcus" accounts for at least 22 times more carbon in these waters and may thus be of much greater significance to the global carbon cycle than "Synechococcus".
pecies
*"S. ambiguus" Skuja
*"S. arcuatus" var. "calcicolus" Fjerdingstad
*"S. bigranulatus" Skuja
*"S. brunneolus" Rabenhorst
*"S. caldarius" Okada
*"S. capitatus" A. E. Bailey-Watts & J. Komárek
*"S. carcerarius" Norris
*"S. elongatus" (Nägeli) Nägeli
*"S. endogloeicus" F. Hindák
*"S. epigloeicus" F. Hindák
*"S. ferrunginosus" Wawrik
*"S. intermedius" Gardner
*"S. koidzumii" Yoneda
*"S. lividus" Copeland
*"S. marinus" Jao
*"S. minutissimus" Negoro
*"S. mundulus" Skuja
*"S. nidulans" (Pringsheim) Komárek
*"S. rayssae" Dor
*"S. rhodobaktron" Komárek & Anagnostidis
*"S. roseo-persicinus" Grunow
*"S. roseo-purpureus" G. S. West
*"S. salinarum" Komárek
*"S. salinus" Frémy
*"S. sciophilus" Skuja
*"S. sigmoideus" (Moore & Carter) Komárek
*"S. spongiarum" Usher "et al."
*"S. subsalsus" Skuja
*"S. sulphuricus" Dor
*"S. vantieghemii" (Pringsheim) Bourrelly
*"S. violaceus" Grunow
*"S. viridissimus" Copeland
*"S. vulcanus" Copelandee also
*
Photosynthetic picoplankton
* "Prochlorococcus "References
Further reading
*cite journal |quotes=no |author=L. Campbell, H. Liu, H. A. Nolla & D. Vaulot |year=1997 |title=Annual variability of phytoplankton and bacteria in the subtropical North Pacific Ocean at Station ALOHA during the 1991-1994 ENSO event |journal=
Deep Sea Research I |volume=44 |pages=167–192 |doi=10.1016/S0967-0637(96)00102-1
*cite journal |quotes=no |author=L. Campbell, H. A. Nolla & D. Vaulot |year=1994 |title=The importance of Prochlorococcus to community structure in the central North Pacific Ocean |journal=Limnology and Oceanography |volume=39 |pages=954–961
*cite journal |quotes=no |author=F. Partensky, J. Blanchot, F. Lantoine, J. Neveux & D. Marie |year=1996 |title=Vertical Structure of Picophytoplankton at Different Trophic Sites of the Tropical Northeastern Atlantic Ocean |journal=Deep Sea Research I |volume=43 |pages=1191–1213 |doi=10.1016/0967-0637(96)00056-8
*cite journal |quotes=no |author=F. Partensky, L. Guillou, N. Simon & D. Vaulot |year=1997 |title=Recent advances in the use of molecular techniques to assess the genetic diversity of marine photosynthetic microorganisms |journal=Vie Milieu |volume=47 |pages=367–374
*cite journal |quotes=no |author=F. Partensky, W. R. Hess & D. Vaulot |year=1999b |title=Prochlorococcus, a Marine Photosynthetic Prokaryote of Global Significance |journal=Microbiology and Molecular Biology Reviews |volume=Mar |pages=106–127
*cite journal |quotes=no |author=F. Partensky, N. Hoepffner, W. K. W. Li, O. Ulloa & D. Vaulot |year=1993 |title=Photoacclimation of Prochlorococcus sp.(Prochlorophyta) strains isolated from the North Atlantic and Mediterranean Sea |journal=Plant Physiology |volume=101 |pages=295–296
*cite book |author=J. B. Waterbury, S. W. Watson, F. W. Valois, D. G. Franks |year=1986a |chapter=Biological and ecological characterization of the marine unicellular cyanobacterium "Synechococcus" |editor=W. K. W. Li |title=Photoynthetic Picoplankton |publisher=Department of Fisheries and Oceans, Ottawa, Canada |pages=71–120
*cite journal |quotes=no |author=J. B. Waterbury & J. M. Willey |year=1988 |title=Isolation and growth of marine planktonic cyanobacteria |journal=Methods in Enzymology |volume=167 |pages=100–105External links
*cite web |author=J. Komárek & M. D. Guiry |title="Synechococcus" Nägeli 1849: 56 |work=
AlgaeBase |url=http://www.algaebase.org/search/genus/detail/?genus_id=43582 |date=2006-07-17
Wikimedia Foundation. 2010.
