- Corey–Fuchs reaction
-
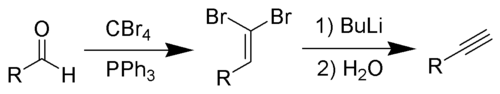
The Corey–Fuchs reaction, also known as the Ramirez–Corey–Fuchs reaction, is a series of chemical reactions designed to transform an aldehyde into an alkyne.[1][2][3] The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was originally discovered by Desai, McKelvie and Ramirez.[4] The overall transformation of an aldehyde to an alkyne by this method is named after its discoverers, American chemists Elias James Corey and Philip L. Fuchs.
By suitable choice of base, it is often possible to stop the reaction at the 1-bromoalkyne, a useful functional group for further transformation.
Reaction mechanism
The Corey–Fuchs reaction is based on a special case of the Wittig Reaction, where the phosphorus ylide is formed from dibromocarbene. This carbene is generated in situ from the reaction of Triphenylphosphine and carbon tetrabromide.

Triphenylphosphine then attacks the nascent carbene to form the reactive ylide. This ylide undergoes a Wittig Reaction when exposed to an aldehyde.

Deprotonation of the weakly acidic olefinic proton with butyllithium gives rise to a lithio-olefinic species which can undergo a beta-elimination to produce the bromoalkyne. Further treatment with butyllithium allows for a lithium–halogen exchange and the intermediate can be quenched with an electrophile, such as water or an alkyl halide, transforming the bromoalkyne to the terminal acetylene, or the internal alkyne, respectively.

References
- ^ Corey, E. J.; Fuchs, P. L. Tetrahedron Lett. 1972, 13, 3769–3772. doi:10.1016/S0040-4039(01)94157-7
- ^ Mori, M.; Tonogaki, K.; Kinoshita, A. Organic Syntheses, Vol. 81, p.1 (2005). (Article)
- ^ Marshall, J. A.; Yanik, M. M.; Adams, N. D.; Ellis, K. C.; Chobanian, H. R. Organic Syntheses, Vol. 81, p.157 (2005). (Article)
- ^ N. B. Desai, N. McKelvie, F. Ramirez JACS, Vol. 84, p.1745-1747 (1962). doi:10.1021/ja00868a057
See also
Categories:- Carbon-carbon bond forming reactions
- Rearrangement reactions
- Name reactions
Wikimedia Foundation. 2010.